Named after the beautiful Cecret lake
Location: 40.570°N 111.622°W , Elevation: 9,875 feet (3,010 m), Hiking level: easy
 |
 |
 |
 |
(Image credit: Intermountain Healthcare)
Table of Contents:
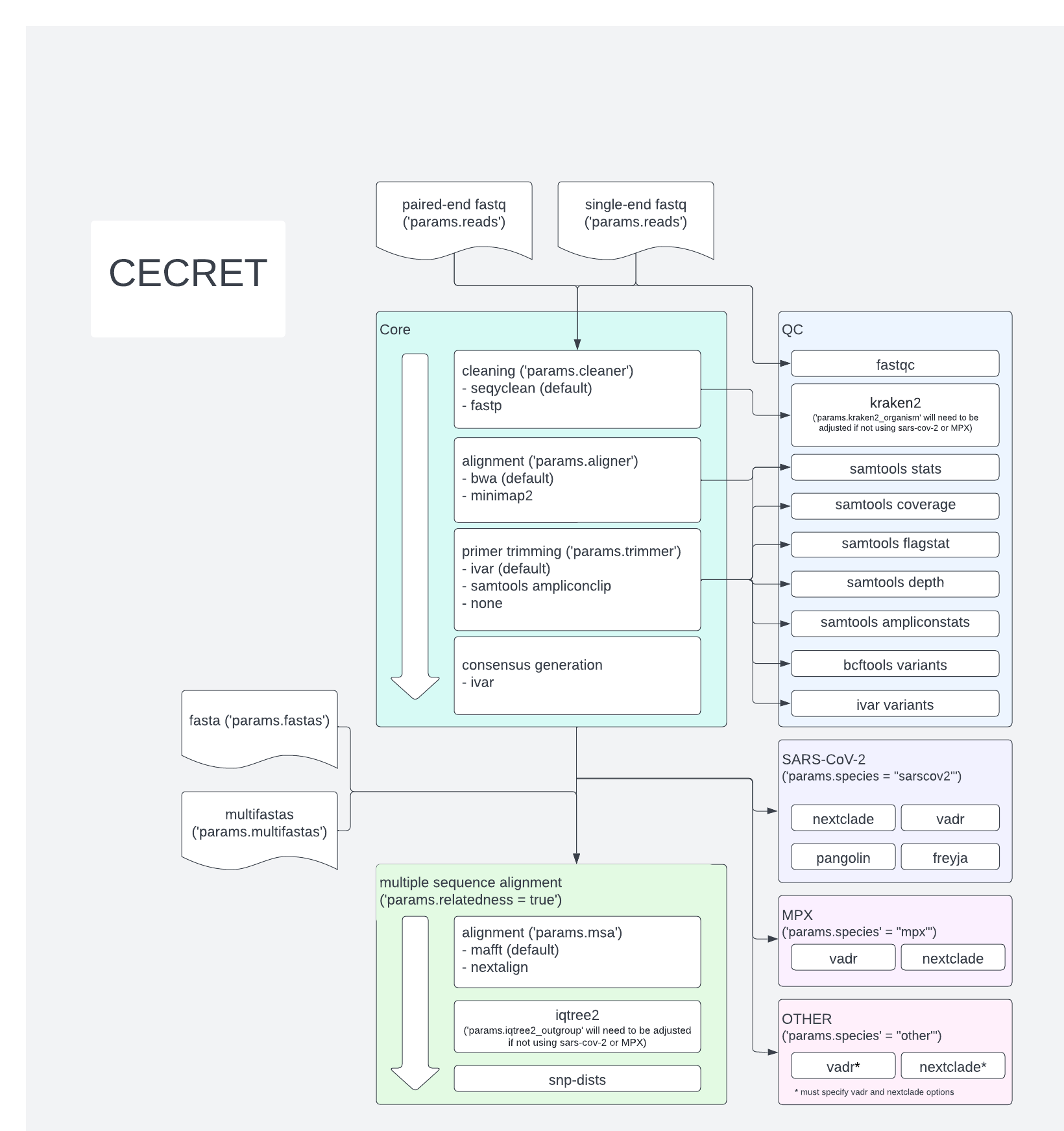
- Introduction
- Dependencies
- Usage
- Input and output directories
- Quality Assessment
- Full workflow
- Determining primer and amplicon bedfiles
- Using the included nextclade dataset
- Determining CPU usage
- Determining depth for base calls
- Determining if duplicates should be taken into account
- SARS-CoV-2 Wastewater
- Monkeypox
- Updating Cecret
- Optional toggles:
- Determining relatedness or creating trees
- Classified reads with Kraken2
- Main components
- Turning off unneeded processes
- Final file structure
- Config files
- Frequently Asked Questions (aka FAQ)
Cecret was originally developed by @erinyoung at the Utah Public Health Laborotory for SARS-COV-2 sequencing with the artic/Illumina hybrid library prep workflow for MiSeq data with protocols here and here. This nextflow workflow, however, is flexible for many additional organisms and primer schemes as long as the reference genome is "small" and "good enough." In 2022, @tives82 added in contributions for Monkeypox virus, including converting IDT's primer scheme to NC_063383.1 coordinates. We are grateful to everyone that has contributed to this repo.
The nextflow workflow was built to work on linux-based operating systems. Additional config options are needed for cloud batch usage.
The library preparation method greatly impacts which bioinformatic tools are recommended for creating a consensus sequence. For example, amplicon-based library prepation methods will required primer trimming and an elevated minimum depth for base-calling. Some bait-derived library prepation methods have a PCR amplification step, and PCR duplicates will need to be removed. This has added complexity and several (admittedly confusing) options to this workflow. Please submit an issue if/when you run into issues.
It is possible to use this workflow to simply annotate fastas generated from any workflow or downloaded from GISAID or NCBI. There are also options for multiple sequence alignment (MSA) and phylogenetic tree creation from the fasta files.
Cecret is also part of the staphb-toolkit.
- Nextflow
- Singularity or Docker - set the profile as singularity or docker during runtime
This workflkow does not run without input files, and there are multiple ways to specify which input files should be used
- using files in directories
- using files specified in a sample sheet
- using params, directories and files specified in a config file
# using singularity on paired-end reads in a directory called 'reads'
nextflow run UPHL-BioNGS/Cecret -profile singularity --reads <directory to reads>
# using docker on samples specified in SampleSheet.csv
nextflow run UPHL-BioNGS/Cecret -profile docker --sample_sheet SampleSheet.csv
# using a config file containing all inputs
nextflow run UPHL-BioNGS/Cecret -c file.config
Results are roughly organiized into 'params.outdir'/< analysis >/sample.result
A file summarizing all results is found in 'params.outdir'/cecret_results.csv and 'params.outdir'/cecret_results.txt.
Consensus sequences can be found in 'params.outdir'/consensus and end with *.consensus.fa.
(can be adjusted with 'params.reads', 'params.single_reads', and 'params.fastas')
Paired-end fastq.gz (ending with 'fastq', 'fastq.gz', 'fq', or 'fq.gz') reads as follows or designate directory with 'params.reads' or '--reads'
directory
└── reads
└── *fastq.gz
WARNING : Sometimes nextflow does not catch every name of paired-end fastq files. This workflow is meant to be fairly agnostic, but if paired-end fastq files are not being found it might be worth renaming them to some sort of sample_1.fastq.gz format or using a sample sheet.
Single-end fastq.gz reads as follows or designate directory with 'params.single_reads' or '--single_reads'
directory
└── single_reads
└── *fastq.gz
WARNING : single and paired-end reads cannot be in the same directory
Fasta files (ending with 'fa', 'fasta', or 'fna') as follows or designate directory with 'params.fastas' or '--fastas'
directory
└── fastas
└── *fasta
MultiFasta files (ending with 'fa', 'fasta', or 'fna') as follows or designate directory with 'params.multifastas' or '--multifastas'
directory
└── multifastas
└── *fasta
WARNING : fastas and multifastas cannot be in the same directory. If no fasta preprocessing is necessary, put the single fastas in the multifastas directory.
Cecret can also use a sample sheet for input with the sample name and reads separated by commas. The header must be sample,fastq_1,fastq_1. The general rule is the identifier for the file(s), the file locations, and the type if not paired-end fastq files.
Rows match files with their processing needs.
- paired-end reads:
sample,read1.fastq.gz,read2.fastq.gz - single-reads reads:
sample,read1.fastq.gz,single - fasta files:
sample,sample.fasta,fasta - multifasta files:
multifasta,multifasta.fasta,multifasta
Example sample sheet:
sample,fastq_1,fastq_2
SRR13957125,/home/eriny/sandbox/test_files/cecret/reads/SRR13957125_1.fastq.gz,/home/eriny/sandbox/test_files/cecret/reads/SRR13957125_2.fastq.gz
SRR13957170,/home/eriny/sandbox/test_files/cecret/reads/SRR13957170_1.fastq.gz,/home/eriny/sandbox/test_files/cecret/reads/SRR13957170_2.fastq.gz
SRR13957177S,/home/eriny/sandbox/test_files/cecret/single_reads/SRR13957177_1.fastq.gz,single
OQ255990.1,/home/eriny/sandbox/test_files/cecret/fastas/OQ255990.1.fasta,fasta
Example usage with sample sheet using docker to manage containers
nextflow run UPHL-BioNGS/Cecret -profile docker --sample_sheet SampleSheet.csv
The default primer scheme of the 'Cecret' workflow is the 'V4' primer scheme developed by artic network for SARS-CoV-2. Releases prior to and including '2.2.20211221' used the 'V3' primer scheme as the default. As many public health laboratories are still using 'V3', the 'V3' files are still in this repo, but now the 'V4', 'V4.1' ('V4' with a spike-in of additional primers), and 'V5.3.2' are also included. The original primer and amplicon bedfiles can be found at artic's github repo.
Setting primers sets is with the corresponding profile.
# using artic V3 primers
nextflow run UPHL-BioNGS/Cecret -profile singularity,artic_V3
# using artic V4 primers
nextflow run UPHL-BioNGS/Cecret -profile singularity,artic_V4
# using artic V4.1 primers
nextflow run UPHL-BioNGS/Cecret -profile singularity,artic_V4_1
# using artic V5.3.2 primers
nextflow run UPHL-BioNGS/Cecret -profile singularity,artic_V5_3_2
Setting primers with a parameter on the command line (these can also be defined in a config file)
# using artic V3 primers
nextflow run UPHL-BioNGS/Cecret -profile singularity --primer_set 'ncov_V3'
# using artic V4 primers
nextflow run UPHL-BioNGS/Cecret -profile singularity --primer_set 'ncov_V4'
# using artic V4.1 primers
nextflow run UPHL-BioNGS/Cecret -profile singularity --primer_set 'ncov_V4.1'
# using artic V5.3.2 primers
nextflow run UPHL-BioNGS/Cecret -profile singularity --primer_set 'ncov_V5.3.2'
It is still possible to set 'params.primer_bed' and 'params.amplicon_bed' via the command line or in a config file with the path to the corresponding file.
It has been requested by some of our more sofisticated colleagues to include a way to upload a nextclade dataset separately. We expect that is mostly for cloud usage. To accomadate this, there is now a sars.zip file in data with a nextclade dataset. To use this included dataset, params.download_nextclade_dataset must be set to false in either the command line of in a config file.
nextflow run UPHL-BioNGS/Cecret -profile singularity --sample_sheet input.csv --download_nextclade_dataset false
This included dataset, however, will only be as current as Cecret's maintainers are able to upload it. There is a Github actions that should attempt to update the nextclade dataset every Tuesday, but this still has be merged and go through testing. The end user can also create a nextclade dataset, and then feed that into this workflow with params.predownloaded_nextclade_dataset.
To create the nextclade dataset with nextclade
nextclade dataset get --name sars-cov-2 --output-zip sars.zip
To use with Cecret
nextflow run UPHL-BioNGS/Cecret -profile singularity --sample_sheet input.csv --download_nextclade_dataset false --predownloaded_nextclade_dataset sars.zip
Or the corresponding params can be set in a config file.
For the sake of simplicity, processes in this workflow are designated 1 CPU, a medium amount of CPUs (5), or the largest amount of CPUs (the number of CPUs of the environment launching the workflow if using the main workflow and a simple config file or 8 if using profiles and the config template). The medium amount of CPUs can be adjusted by the End User by adjusting 'params.medcpus', the largest amount can be adjusted with 'params.maxcpus', or the cpus can be specified for each process individually in a config file.
The End User can adjust this by specifying the maximum cpus that one process can take in the config file 'params.maxcpus = <new value>' or on the command line
nextflow run UPHL-BioNGS/Cecret -profile singularity --maxcpus <new value>
It is important to remember that nextflow will attempt to utilize all CPUs available, and this value is restricted to one process. As a specific example, the prcoess 'bwa' will be allocated 'params.maxcpus'. If there are 48 CPUs available and 'params.maxcpus = 8', then 6 samples can be run simultaneously.
Sequencing has an intrinsic amount of error for every predicted base on a read. This error is reduced the more reads there are. As such, there is a minimum amount of depth that is required to call a base with ivar consensus, ivar variants, and bcftools variants. The main assumption of using this workflow is that the virus is clonal (i.e. only one infection represented in a sample) and created via pcr amplified libraries. The default depth for calling bases or finding variants is set with 'params.minimum_depth' with the default value being 'params.minimum_depth = 100'. This parameter can be adjusted by the END USER in a config file or on the command line.
A corresponding parameter is 'params.mpileup_depth' (default of 'params.mpileup_depth = 8000'), which is the number of reads that samtools (used by ivar) or bcftools uses to put into memory for any given position. If the END USER is experiencing memory issues, this number may need to be decreased.
For library preparation methods with baits followed by PCR amplification, it is recommended to remove duplicate reads. For most other methods, removing deplicates will generally not harm anything. To remove duplicates, set the 'params.markdup' to true. This removes duplicate reads from the aligned sam file, which is before the primer trimming and after the filter processes. This will likely enable a lower minimum depth for variant calling (default is 100).
On the command line:
nextflow run UPHL-BioNGS/Cecret -profile singularity --markdup true --minimum_depth 10
In a config file:
params.markdup = true
params.minimum_depth = 10
The defaults for Cecret continue to be for SARS-CoV-2, but there are growing demands for a workflow for Monkeypox Virus. As such, there are a few parameters that might benefit the End User.
There are three profiles for Monkeypox Virus sequencing : mpx, mpx_idt and mpx_primalseq. The mpx profile has some defaults for a metagenomic-type sequencing, while mpx_idt is for libraries prepped with IDT's primers, and mpx_primalseq which has been validated with Illumina library prep methods and sequencing platforms.
# metagenomic
nextflow run UPHL-BioNGS/Cecret -profile singularity,mpx
# using IDT's primers
nextflow run UPHL-BioNGS/Cecret -profile singularity,mpx_idt
# using Illumina library prep methods and sequencing platforms
nextflow run UPHL-BioNGS/Cecret -profile singularity,mpx_primalseq
There are amplicon-based methods, bait, and amplicon-bait hybrid library preparation methods which increases the portion of reads for a relevant organism. If there is a common preparation for the End User, please submit an issue, and we can create a profile or config file. Remember that the bedfiles for the primer schemes and amplicons MUST match the reference.
This workflow has also been used with primer-amplified Illumina sequencing of wastewater. Patient samples conceptually are different than wastewater samples, but many of the bioinformatic steps are the same. The files from Freyja are likely the most significant for this analysis. Freyja uses the bam files after primer trimming has been completed to look for variants and their proportions to assign expected pangolin lineages.
Recommended parameter adjustements for wastewater
params.species = 'sarscov2' //this is the default, but it is required for the subworkflow that involves freyja
params.bcftools_variants = false
params.ivar_variants = false
params.pangolin = false
params.nextclade = false
params.vadr = false
Pangolin, Nextclade, and any analysis that evaluates the consensus fasta are not as useful in the context of wastewater. There is currently not a way to remove consensus sequence generation from Cecret, but an option may be available in the future if there is "enough" demand.
nextflow pull UPHL-BioNGS/Cecret
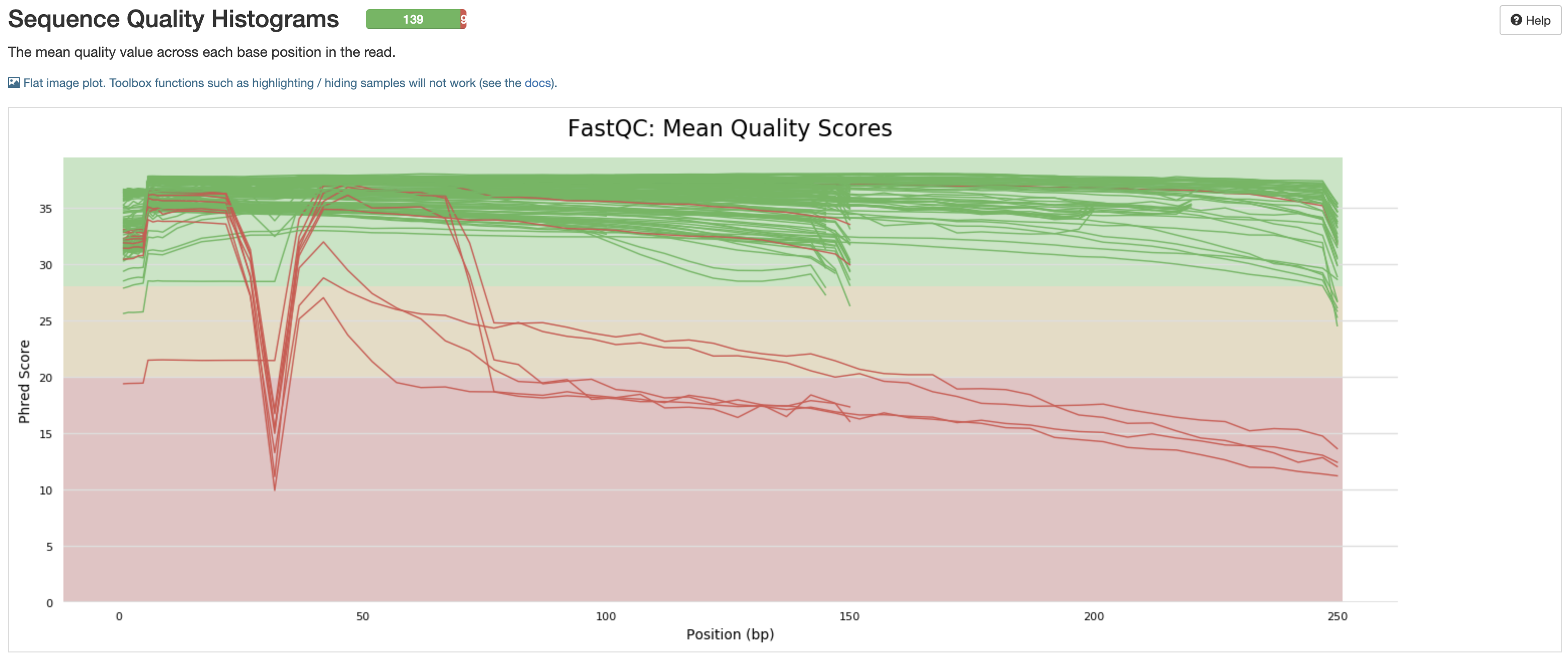
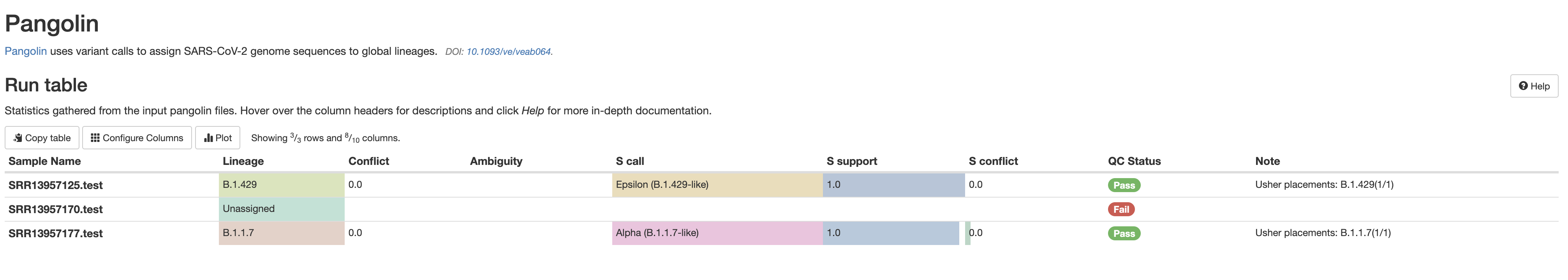
The quality of a sequencing run is very important. As such, many values are recorded so that the End User can assess the quality of the results produced from a sequencing run.
fastqc_raw_reads_1andfastqc_raw_reads_2are the number of reads prior to cleaning by either seqyclean or fastp.seqyclean_Perc_Kept(params.cleaner = 'seqyclean') orfastp_pct_surviving(params.cleaner = 'fastp') indicate how many reads remain after removal of low-quality reads (more = better).num_Nis the number of uncalled bases in the generated consensus sequence (less = better).num_totalis the total number of called bases in the generated consensus sequequence (more = better). As many consensus sequences are generated with this workflow via amplicon sequencing, the intitial and end of the reference often has little coverage. This means that the number of bases in the consensus sequence is less than the length of the reference sequence.num_pos_${params.minimum_depth}X(which isnum_pos_100Xby default) is the number of positions for which there is sufficient depth to call variants (more = better). Any sequence below this value will be anN.aci_num_failed_ampliconsuses the amplicon file to give a rough estimate as to which primer pairs are not getting enough coverage (less = better).samtools_num_failed_ampliconsuses the primer file to detect primer pairs and estimates coverages based on this (less = better).
More imformation on evaluating amplicon/primer failure can be found in the FAQ under the question 'Is there a way to determine if certain amplicons are failing?'
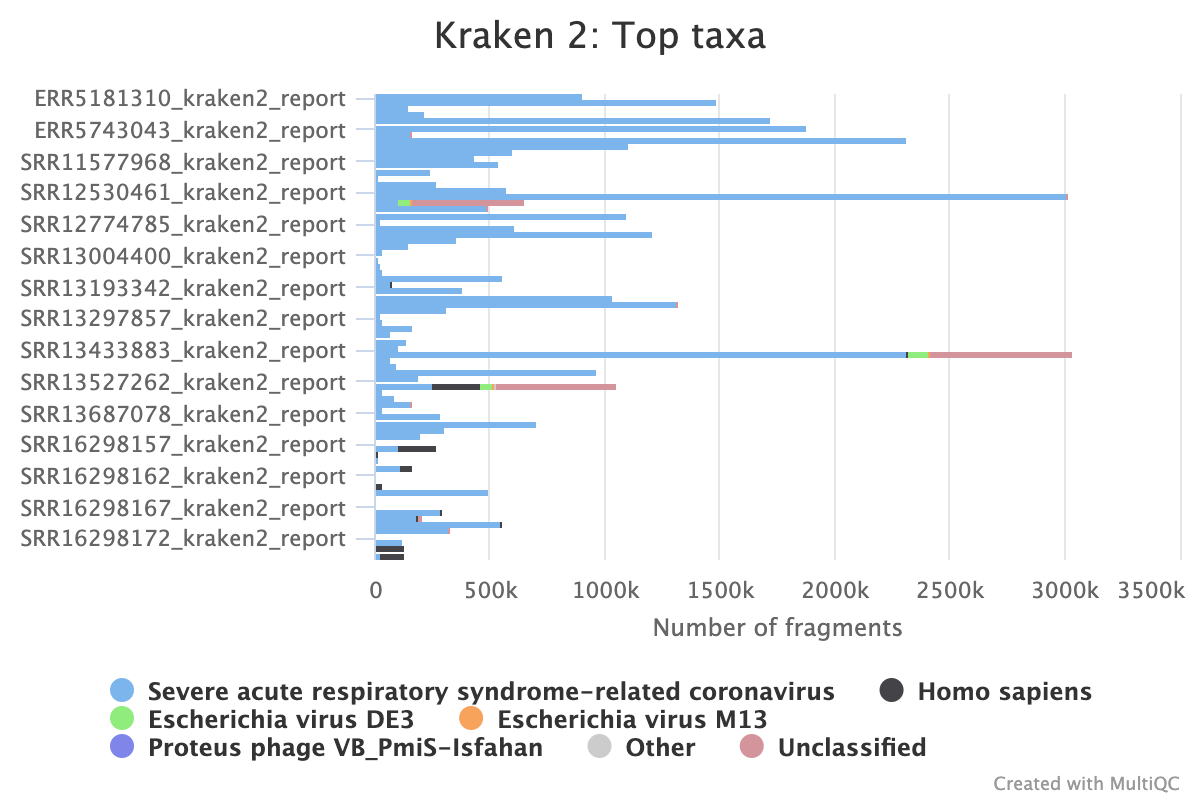
Kraken2 is optional for this workflow, but can provide additional quality assessment metrics:
top_organismis the most common organism identified in the reads.percent_reads_top_organismis the percentage of reads assigned that organism (more = better).%_human_readsis the percentage of human reads reads (less = better).
nextflow run UPHL-BioNGS/Cecret -profile singularity --cleaner fastp
Or set params.cleaner = 'fastp' in a config file
nextflow run UPHL-BioNGS/Cecret -profile singularity --trimmer samtools
Or set params.trimmer = 'samtools' in a config file
nextflow run UPHL-BioNGS/Cecret -profile singularity --trimmer none
Or set params.trimmer = 'none' in a config file
nextflow run UPHL-BioNGS/Cecret -profile singularity --aligner minimap2
Or set params.aligner = 'minimap2' in a config file
To create a multiple sequence alignment and corresponding phylogenetic tree and SNP matrix, set params.relatedness = true or
nextflow run UPHL-BioNGS/Cecret -profile singularity --relatedness true
nextflow run UPHL-BioNGS/Cecret -profile singularity --relatedness true --msa nextalign
Or set params.msa = 'nextalign' and params.relatedness = true in a config file
And then you get trees like this which can visualized with itol or ggtree.

To classify reads with kraken2 to identify reads from human or the organism of choice
mkdir kraken2_db
cd kraken2_db
wget https://genome-idx.s3.amazonaws.com/kraken/k2_standard_08gb_20230605.tar.gz
tar -zxvf minikraken2_v2_8GB_201904.tgz
params.kraken2 = true
params.kraken2_db = 'kraken2_db'
- aci - for depth estimation over amplicons
- bwa - for aligning reads to the reference
- fastp - for cleaning reads ; optional, faster alternative to seqyclean
- fastqc - for QC metrics
- freyja - for multiple SARS-CoV-2 lineage classifications
- iqtree2 - for phylogenetic tree generation (optional, relatedness must be set to "true")
- ivar - calling variants and creating a consensus fasta; optional primer trimmer
- kraken2 - for read classification
- mafft - for multiple sequence alignment (optional, relatedness must be set to "true")
- minimap2 - an alternative to bwa
- multiqc - summary of results
- nextalign - for phylogenetic tree generation (optional, relatedness must be set to "true", and msa must be set to "nextalign")
- nextclade - for SARS-CoV-2 clade classification
- pangolin - for SARS-CoV-2 lineage classification
- samtools - for QC metrics and sorting; optional primer trimmer; optional converting bam to fastq files; optional duplication marking
- seqyclean - for cleaning reads
- snp-dists - for relatedness determination (optional, relatedness must be set to "true")
- vadr - for annotating fastas like NCBI
It came to my attention that some processes (like bcftools) do not work consistently. Also, they might take longer than wanted and might not even be needed for the end user. Here's the processes that can be turned off with their default values:
params.bcftools_variants = true # vcf of variants
params.fastqc = true # qc on the sequencing reads
params.ivar_variants = true # itemize the variants identified by ivar
params.samtools_stats = true # stats about the bam files
params.samtools_coverage = true # stats about the bam files
params.samtools_depth = true # stats about the bam files
params.samtools_flagstat = true # stats about the bam files
params.samtools_ampliconstats = true # stats about the amplicons
params.samtools_plot_ampliconstats = true # images related to amplicon performance
params.kraken2 = false # used to classify reads and needs a corresponding params.kraken2_db and organism if not SARS-CoV-2
params.aci = true # coverage approximation of amplicons
params.nextclade = true # SARS-CoV-2 clade determination
params.pangolin = true # SARS-CoV-2 lineage determination
params.freyja = true # multiple SARS-CoV-2 lineage determination
params.vadr = false # NCBI fasta QC
params.relatedness = false # create multiple sequence alignments with input fastq and fasta files
params.snpdists = true # creates snp matrix from mafft multiple sequence alignment
params.iqtree2 = true # creates phylogenetic tree from mafft multiple sequence alignement
params.bamsnap = false # has been removed
params.rename = false # needs a corresponding sample file and will rename files for GISAID and NCBI submission
params.filter = false # takes the aligned reads and turns them back into fastq.gz files
params.multiqc = true # aggregates data into single report
Final File Tree after running cecret.nf
cecret # results from this workflow
├── aligned # aligned (with aligner) but untrimmed bam files with indexes
│ ├── SRR13957125.sorted.bam
│ ├── SRR13957125.sorted.bam.bai
│ ├── SRR13957170.sorted.bam
│ ├── SRR13957170.sorted.bam.bai
│ ├── SRR13957177.sorted.bam
│ └── SRR13957177.sorted.bam.bai
├── bcftools_variants # set to false by default; VCF files of variants identified
│ ├── SRR13957125.vcf
│ ├── SRR13957170.vcf
│ └── SRR13957177.vcf
├── cecret_results.csv # comma-delimeted summary of results
├── cecret_results.txt # tab-delimited summary of results
├── consensus # the likely reason you are running this workflow
│ ├── SRR13957125.consensus.fa
│ ├── SRR13957170.consensus.fa
│ └── SRR13957177.consensus.fa
├── dataset # generated by nextclade
│ ├── genemap.gff
│ ├── primers.csv
│ ├── qc.json
│ ├── reference.fasta
│ ├── sequences.fasta
│ ├── tag.json
│ ├── tree.json
│ └── virus_properties.json
├── fasta_prep # optional for inputted fastas
│ ├── SRR13957125.test.fa
│ ├── SRR13957170.test.fa
│ └── SRR13957177.test.fa
├── fastp # optional tools for cleaning reads when 'params.cleaner = fastp'
│ ├── SRR13957125_clean_PE1.fastq.gz
│ ├── SRR13957125_clean_PE2.fastq.gz
│ ├── SRR13957125_fastp.html
│ ├── SRR13957125_fastp.json
│ ├── SRR13957170_clean_PE1.fastq.gz
│ ├── SRR13957170_clean_PE2.fastq.gz
│ ├── SRR13957170_fastp.html
│ ├── SRR13957170_fastp.json
│ ├── SRR13957177_clean_PE1.fastq.gz
│ ├── SRR13957177_clean_PE2.fastq.gz
│ ├── SRR13957177_fastp.html
│ └── SRR13957177_fastp.json
├── fastqc # QC metrics for each fasta sequence
│ ├── SRR13957125_1_fastqc.html
│ ├── SRR13957125_1_fastqc.zip
│ ├── SRR13957125_2_fastqc.html
│ ├── SRR13957125_2_fastqc.zip
│ ├── SRR13957170_1_fastqc.html
│ ├── SRR13957170_1_fastqc.zip
│ ├── SRR13957170_2_fastqc.html
│ ├── SRR13957170_2_fastqc.zip
│ ├── SRR13957177_1_fastqc.html
│ ├── SRR13957177_1_fastqc.zip
│ ├── SRR13957177_2_fastqc.html
│ └── SRR13957177_2_fastqc.zip
├── filter # fastq.gz files from reads that were aligned to the reference genome
│ ├── SRR13957125_filtered_R1.fastq.gz
│ ├── SRR13957125_filtered_R2.fastq.gz
│ ├── SRR13957125_filtered_unpaired.fastq.gz
│ ├── SRR13957170_filtered_R1.fastq.gz
│ ├── SRR13957170_filtered_R2.fastq.gz
│ ├── SRR13957170_filtered_unpaired.fastq.gz
│ ├── SRR13957177_filtered_R1.fastq.gz
│ ├── SRR13957177_filtered_R2.fastq.gz
│ └── SRR13957177_filtered_unpaired.fastq.gz
├── freyja # finding co-lineages
│ ├── aggregated-freyja.png
│ ├── aggregated-freyja.tsv
│ ├── SRR13957125_boot.tsv_lineages.csv
│ ├── SRR13957125_boot.tsv_summarized.csv
│ ├── SRR13957125_demix.tsv
│ ├── SRR13957125_depths.tsv
│ ├── SRR13957125_variants.tsv
│ ├── SRR13957170_boot.tsv_lineages.csv
│ ├── SRR13957170_boot.tsv_summarized.csv
│ ├── SRR13957170_demix.tsv
│ ├── SRR13957170_depths.tsv
│ ├── SRR13957170_variants.tsv
│ ├── SRR13957177_boot.tsv_lineages.csv
│ ├── SRR13957177_boot.tsv_summarized.csv
│ ├── SRR13957177_demix.tsv
│ ├── SRR13957177_depths.tsv
│ └── SRR13957177_variants.tsv
├── iqtree2 # phylogenetic tree that is generated with 'params.relatedness = true'
│ ├── iqtree2.iqtree
│ ├── iqtree2.log
│ ├── iqtree2.mldist
│ └── iqtree2.treefile
├── ivar_trim # bam files after primers have been trimmed off the reads with ivar
│ ├── SRR13957125_ivar.log
│ ├── SRR13957125.primertrim.sorted.bam
│ ├── SRR13957125.primertrim.sorted.bam.bai
│ ├── SRR13957170_ivar.log
│ ├── SRR13957170.primertrim.sorted.bam
│ ├── SRR13957170.primertrim.sorted.bam.bai
│ ├── SRR13957177_ivar.log
│ ├── SRR13957177.primertrim.sorted.bam
│ └── SRR13957177.primertrim.sorted.bam.bai
├── ivar_variants # tsv and vcf files of variants identified in sample
│ ├── SRR13957125.ivar_variants.vcf
│ ├── SRR13957125.variants.tsv
│ ├── SRR13957170.ivar_variants.vcf
│ ├── SRR13957170.variants.tsv
│ ├── SRR13957177.ivar_variants.vcf
│ └── SRR13957177.variants.tsv
├── kraken2 # kraken2 report of the organisms the reads may be from
│ ├── SRR13957125_kraken2_report.txt
│ ├── SRR13957170_kraken2_report.txt
│ └── SRR13957177_kraken2_report.txt
├── logs # divided log and err files for QC and troubleshooting pleasures
│ └── processes*
│ ├── sample.run_id.err
│ └── sample.run_id.log
├── mafft # multiple sequence alignment created when 'params.relatedness = true'
│ └── mafft_aligned.fasta
├── markdup
│ ├── SRR13957125.markdup.sorted.bam
│ ├── SRR13957125.markdup.sorted.bam.bai
│ ├── SRR13957125_markdupstats.txt
│ ├── SRR13957170.markdup.sorted.bam
│ ├── SRR13957170.markdup.sorted.bam.bai
│ ├── SRR13957170_markdupstats.txt
│ ├── SRR13957177.markdup.sorted.bam
│ ├── SRR13957177.markdup.sorted.bam.bai
│ └── SRR13957177_markdupstats.txt
├── multiqc # aggregates data into single report
│ ├── multiqc_data
│ │ ├── multiqc_citations.txt
│ │ ├── multiqc_data.json
│ │ ├── multiqc_fastqc.txt
│ │ ├── multiqc_general_stats.txt
│ │ ├── multiqc_ivar_primers.txt
│ │ ├── multiqc_ivar_summary.txt
│ │ ├── multiqc.log
│ │ ├── multiqc_samtools_flagstat.txt
│ │ ├── multiqc_samtools_stats.txt
│ │ ├── multiqc_seqyclean.txt
│ │ └── multiqc_sources.txt
│ └── multiqc_report.html
├── nextclade # nextclade reports
│ ├── combined.fasta
│ ├── nextclade.aligned.fasta
│ ├── nextclade.auspice.json
│ ├── nextclade.csv
│ ├── nextclade.errors.csv
│ ├── nextclade.gene.E.fasta
│ ├── nextclade.gene.M.fasta
│ ├── nextclade.gene.N.fasta
│ ├── nextclade.gene.ORF1a.fasta
│ ├── nextclade.gene.ORF1b.fasta
│ ├── nextclade.gene.ORF3a.fasta
│ ├── nextclade.gene.ORF6.fasta
│ ├── nextclade.gene.ORF7a.fasta
│ ├── nextclade.gene.ORF7b.fasta
│ ├── nextclade.gene.ORF8.fasta
│ ├── nextclade.gene.ORF9b.fasta
│ ├── nextclade.gene.S.fasta
│ ├── nextclade.insertions.csv
│ ├── nextclade.json
│ └── nextclade.tsv
├── pangolin # pangolin results
│ ├── combined.fasta
│ └── lineage_report.csv
├── samtools_ampliconstats # amplicon statistics and metrics as determined by samtools
│ ├── SRR13957125_ampliconstats.txt
│ ├── SRR13957170_ampliconstats.txt
│ └── SRR13957177_ampliconstats.txt
├── samtools_coverage # coverage and metrics as determined by samtools
│ ├── SRR13957125.cov.aligned.hist
│ ├── SRR13957125.cov.aligned.txt
│ ├── SRR13957125.cov.trimmed.hist
│ ├── SRR13957125.cov.trimmed.txt
│ ├── SRR13957170.cov.aligned.hist
│ ├── SRR13957170.cov.aligned.txt
│ ├── SRR13957170.cov.trimmed.hist
│ ├── SRR13957170.cov.trimmed.txt
│ ├── SRR13957177.cov.aligned.hist
│ ├── SRR13957177.cov.aligned.txt
│ ├── SRR13957177.cov.trimmed.hist
│ └── SRR13957177.cov.trimmed.txt
├── samtools_depth # the number of reads
│ ├── SRR13957125.depth.aligned.txt
│ ├── SRR13957125.depth.trimmed.txt
│ ├── SRR13957170.depth.aligned.txt
│ ├── SRR13957170.depth.trimmed.txt
│ ├── SRR13957177.depth.aligned.txt
│ └── SRR13957177.depth.trimmed.txt
├── samtools_flagstat # flag information
│ ├── SRR13957125.flagstat.aligned.txt
│ ├── SRR13957125.flagstat.trimmed.txt
│ ├── SRR13957125.flagstat.txt
│ ├── SRR13957170.flagstat.aligned.txt
│ ├── SRR13957170.flagstat.trimmed.txt
│ ├── SRR13957170.flagstat.txt
│ ├── SRR13957177.flagstat.aligned.txt
│ ├── SRR13957177.flagstat.trimmed.txt
│ └── SRR13957177.flagstat.txt
├── samtools_plot_ampliconstats # plots of the ampliconstats for troubleshooting purposes
│ ├── SRR13957125
│ ├── SRR13957125-combined-amp.gp
│ ├── SRR13957125-combined-amp.png
│ ├── SRR13957125-combined-coverage-1.gp
│ ├── SRR13957125-combined-coverage-1.png
│ ├── SRR13957125-combined-depth.gp
│ ├── SRR13957125-combined-depth.png
│ ├── SRR13957125-combined-read-perc.gp
│ ├── SRR13957125-combined-read-perc.png
│ ├── SRR13957125-combined-reads.gp
│ ├── SRR13957125-combined-reads.png
│ ├── SRR13957125-combined-tcoord.gp
│ ├── SRR13957125-combined-tcoord.png
│ ├── SRR13957125-combined-tdepth.gp
│ ├── SRR13957125-combined-tdepth.png
│ ├── SRR13957125-heat-amp-1.gp
│ ├── SRR13957125-heat-amp-1.png
│ ├── SRR13957125-heat-coverage-1-1.gp
│ ├── SRR13957125-heat-coverage-1-1.png
│ ├── SRR13957125-heat-read-perc-1.gp
│ ├── SRR13957125-heat-read-perc-1.png
│ ├── SRR13957125-heat-read-perc-log-1.gp
│ ├── SRR13957125-heat-read-perc-log-1.png
│ ├── SRR13957125-heat-reads-1.gp
│ ├── SRR13957125-heat-reads-1.png
│ ├── SRR13957125-SRR13957125.primertrim.sorted-amp.gp
│ ├── SRR13957125-SRR13957125.primertrim.sorted-amp.png
│ ├── SRR13957125-SRR13957125.primertrim.sorted-cov.gp
│ ├── SRR13957125-SRR13957125.primertrim.sorted-cov.png
│ ├── SRR13957125-SRR13957125.primertrim.sorted-reads.gp
│ ├── SRR13957125-SRR13957125.primertrim.sorted-reads.png
│ ├── SRR13957125-SRR13957125.primertrim.sorted-tcoord.gp
│ ├── SRR13957125-SRR13957125.primertrim.sorted-tcoord.png
│ ├── SRR13957125-SRR13957125.primertrim.sorted-tdepth.gp
│ ├── SRR13957125-SRR13957125.primertrim.sorted-tdepth.png
│ ├── SRR13957125-SRR13957125.primertrim.sorted-tsize.gp
│ ├── SRR13957125-SRR13957125.primertrim.sorted-tsize.png
│ ├── SRR13957170
│ ├── SRR13957170-combined-amp.gp
│ ├── SRR13957170-combined-amp.png
│ ├── SRR13957170-combined-coverage-1.gp
│ ├── SRR13957170-combined-coverage-1.png
│ ├── SRR13957170-combined-depth.gp
│ ├── SRR13957170-combined-depth.png
│ ├── SRR13957170-combined-read-perc.gp
│ ├── SRR13957170-combined-read-perc.png
│ ├── SRR13957170-combined-reads.gp
│ ├── SRR13957170-combined-reads.png
│ ├── SRR13957170-combined-tdepth.gp
│ ├── SRR13957170-combined-tdepth.png
│ ├── SRR13957170-heat-amp-1.gp
│ ├── SRR13957170-heat-amp-1.png
│ ├── SRR13957170-heat-coverage-1-1.gp
│ ├── SRR13957170-heat-coverage-1-1.png
│ ├── SRR13957170-heat-read-perc-1.gp
│ ├── SRR13957170-heat-read-perc-1.png
│ ├── SRR13957170-heat-read-perc-log-1.gp
│ ├── SRR13957170-heat-read-perc-log-1.png
│ ├── SRR13957170-heat-reads-1.gp
│ ├── SRR13957170-heat-reads-1.png
│ ├── SRR13957170-SRR13957170.primertrim.sorted-amp.gp
│ ├── SRR13957170-SRR13957170.primertrim.sorted-amp.png
│ ├── SRR13957170-SRR13957170.primertrim.sorted-cov.gp
│ ├── SRR13957170-SRR13957170.primertrim.sorted-cov.png
│ ├── SRR13957170-SRR13957170.primertrim.sorted-reads.gp
│ ├── SRR13957170-SRR13957170.primertrim.sorted-reads.png
│ ├── SRR13957170-SRR13957170.primertrim.sorted-tdepth.gp
│ ├── SRR13957170-SRR13957170.primertrim.sorted-tdepth.png
│ ├── SRR13957177
│ ├── SRR13957177-combined-amp.gp
│ ├── SRR13957177-combined-amp.png
│ ├── SRR13957177-combined-coverage-1.gp
│ ├── SRR13957177-combined-coverage-1.png
│ ├── SRR13957177-combined-depth.gp
│ ├── SRR13957177-combined-depth.png
│ ├── SRR13957177-combined-read-perc.gp
│ ├── SRR13957177-combined-read-perc.png
│ ├── SRR13957177-combined-reads.gp
│ ├── SRR13957177-combined-reads.png
│ ├── SRR13957177-combined-tcoord.gp
│ ├── SRR13957177-combined-tcoord.png
│ ├── SRR13957177-combined-tdepth.gp
│ ├── SRR13957177-combined-tdepth.png
│ ├── SRR13957177-heat-amp-1.gp
│ ├── SRR13957177-heat-amp-1.png
│ ├── SRR13957177-heat-coverage-1-1.gp
│ ├── SRR13957177-heat-coverage-1-1.png
│ ├── SRR13957177-heat-read-perc-1.gp
│ ├── SRR13957177-heat-read-perc-1.png
│ ├── SRR13957177-heat-read-perc-log-1.gp
│ ├── SRR13957177-heat-read-perc-log-1.png
│ ├── SRR13957177-heat-reads-1.gp
│ ├── SRR13957177-heat-reads-1.png
│ ├── SRR13957177-SRR13957177.primertrim.sorted-amp.gp
│ ├── SRR13957177-SRR13957177.primertrim.sorted-amp.png
│ ├── SRR13957177-SRR13957177.primertrim.sorted-cov.gp
│ ├── SRR13957177-SRR13957177.primertrim.sorted-cov.png
│ ├── SRR13957177-SRR13957177.primertrim.sorted-reads.gp
│ ├── SRR13957177-SRR13957177.primertrim.sorted-reads.png
│ ├── SRR13957177-SRR13957177.primertrim.sorted-tcoord.gp
│ ├── SRR13957177-SRR13957177.primertrim.sorted-tcoord.png
│ ├── SRR13957177-SRR13957177.primertrim.sorted-tdepth.gp
│ ├── SRR13957177-SRR13957177.primertrim.sorted-tdepth.png
│ ├── SRR13957177-SRR13957177.primertrim.sorted-tsize.gp
│ └── SRR13957177-SRR13957177.primertrim.sorted-tsize.png
├── samtools_stats # stats as determined by samtools
│ ├── SRR13957125.stats.aligned.txt
│ ├── SRR13957125.stats.trimmed.txt
│ ├── SRR13957125.stats.txt
│ ├── SRR13957170.stats.aligned.txt
│ ├── SRR13957170.stats.trimmed.txt
│ ├── SRR13957170.stats.txt
│ ├── SRR13957177.stats.aligned.txt
│ ├── SRR13957177.stats.trimmed.txt
│ └── SRR13957177.stats.txt
├── seqyclean # reads that have had PhiX and adapters removed
│ ├── Combined_SummaryStatistics.tsv
│ ├── SRR13957125_clean_PE1.fastq.gz
│ ├── SRR13957125_clean_PE2.fastq.gz
│ ├── SRR13957125_clean_SummaryStatistics.tsv
│ ├── SRR13957125_clean_SummaryStatistics.txt
│ ├── SRR13957170_clean_PE1.fastq.gz
│ ├── SRR13957170_clean_PE2.fastq.gz
│ ├── SRR13957170_clean_SummaryStatistics.tsv
│ ├── SRR13957170_clean_SummaryStatistics.txt
│ ├── SRR13957177_clean_PE1.fastq.gz
│ ├── SRR13957177_clean_PE2.fastq.gz
│ ├── SRR13957177_clean_SummaryStatistics.tsv
│ └── SRR13957177_clean_SummaryStatistics.txt
├── snp-dists # SNP matrix created with 'params.relatedness = true'
│ └── snp-dists.txt
└── vadr # consensus file QC
├── combined.fasta
├── trimmed.fasta
├── vadr.vadr.alc
├── vadr.vadr.alt
├── vadr.vadr.alt.list
├── vadr.vadr.cmd
├── vadr.vadr.dcr
├── vadr.vadr.fail.fa
├── vadr.vadr.fail.list
├── vadr.vadr.fail.tbl
├── vadr.vadr.filelist
├── vadr.vadr.ftr
├── vadr.vadr.log
├── vadr.vadr.mdl
├── vadr.vadr.pass.fa
├── vadr.vadr.pass.list
├── vadr.vadr.pass.tbl
├── vadr.vadr.rpn
├── vadr.vadr.sda
├── vadr.vadr.seqstat
├── vadr.vadr.sgm
├── vadr.vadr.sqa
└── vadr.vadr.sqc
reads # user supplied fastq files for analysis
single_reads # user supplied fastq files for analysis
fastas # user supplied fasta files for analysis
multifastas # user supplied multifasta files for analysis
work # nextflow's working directories
A FILE THAT THE END USER CAN COPY AND EDIT IS FOUND AT configs/cecret_config_template.config
To get a copy of the config file, the End User can use the following command. This creates an edit_me.config file in the current directory.
nextflow run UPHL-BioNGS/Cecret --config_file true
This file contains all of the configurable parameters with their default values. Use '-c' to specify the edited config file.
The End User should not have to change the files in the Cecret repository in order to get the analyses that they need. Nextflow has a wonderful system of config files, which are recommended. Please read nextflow's documentation about config files at https://www.nextflow.io/docs/latest/config.html
The general format of a config file is
param.<parameter that needs adjusting> = <what it needs to be adjusted to>
Then this config file is used with the workflow via the following
nextflow run UPHL-BioNGS/Cecret -c <path to custom config file>
In theory, the values specified in this config file will be used over the defaults set in the workflow.
If the End User is using some sort of cloud or HPC setup, it is highly recommended that this file is copied and edited appropriately. A limited list of parameters is listed below:
- params.reads = workflow.launchDir + '/reads'
- params.single_reads = workflow.launchDir + '/single_reads'
- params.fastas = workflow.launchDir + '/fastas'
- params.multifastas = workflow.launchDir + '/multifastas'
- params.outdir = workflow.launchDir + '/cecret'
- To "resume" a workflow, use
-resumewith the nextflow command - To create a report, use
-with-reportwith the nextflow command - To use nextflow tower, use
-with-towerwith the nextflow command (reports will not be available for download from nextflow tower using this method)
TELL US ABOUT IT!!!
- Github issue
- Send someone from UPHL on slack
Be sure to include the command that was used, what config file was used, and what the nextflow error was.
The multiqc report aggregates data across your samples into one file. Open the 'cecret/multiqc/multiqc_report.html' file with your favored browser. There tables and graphs are generated for 'General Statistics', 'Samtools stats', 'Samtools flagstats', 'FastQC', 'iVar', 'SeqyClean', 'Fastp', 'Pangolin', and 'Kraken2'.
In the history of this repository, there actually was an attempt to store fastq files here that the End User could use to test out this workflow. This made the repository very large and difficult to download.
There are several test profiles. These download fastq files from the ENA to use in the workflow. This does not always work due to local internet connectivity issues, but may work fine for everyone else.
nextflow run UPHL-BioNGS/Cecret -profile {docker or singularity},test
Another great resources is SARS-CoV-2 datasets, an effort of the CDC to provide a benchmark dataset for validating bioinformatic workflows. Fastq files from the nonviovoc, voivoc, and failed projects were downloaded from the SRA and put through this workflow and tested locally before releasing a new version.
The expected amount of time to run this workflow with 250 G RAM and 48 CPUs, 'params.maxcpus = 8', and 'params.medcpus = 4' is ~42 minutes. This corresponded with 25.8 CPU hours.
# for a collection of fastas
nextflow run UPHL-BioNGS/Cecret -profile singularity --fastas <directory with fastas>
# for a collection of fastas and multifastas
nextflow run UPHL-BioNGS/Cecret -profile singularity --fastas <directory with fastas> --multifastas <directory with multifastas>
The End User can run mafft, snpdists, and iqtree on a collection of fastas as well with
nextflow run UPHL-BioNGS/Cecret -profile singularity --relatedness true --fastas <directory with fastas> --multifastas <directory with multifastas>
The End User can have paired-end, singled-end, and fastas that can all be put together into one analysis.
nextflow run UPHL-BioNGS/Cecret -profile singularity --relatedness true --fastas <directory with fastas> --multifastas <directory with multifastas> --reads <directory with paire-end reads> --single_reads <directory with single-end reads>
The End User is more than welcome to look at an example here. Just remove the comments for the parameters that need to be adjusted and specify with -c.
To get a copy of the config file, the End User can use the following command. This created edit_me.config in the current directory.
nextflow run UPHL-BioNGS/Cecret --config_file true
At UPHL, our config file is small enough to be put as a profile option, but the text of the config file would be as follows:
singularity.enabled = true
singularity.autoMounts = true
params {
reads = "Sequencing_reads/Raw"
kraken2 = true
kraken2_db = '/Volumes/IDGenomics_NAS/Data/kraken2_db/h+v'
vadr = false
}
And then run with
nextflow run UPHL-BioNGS/Cecret -c <path to custom config file>
There are two ways to do this.
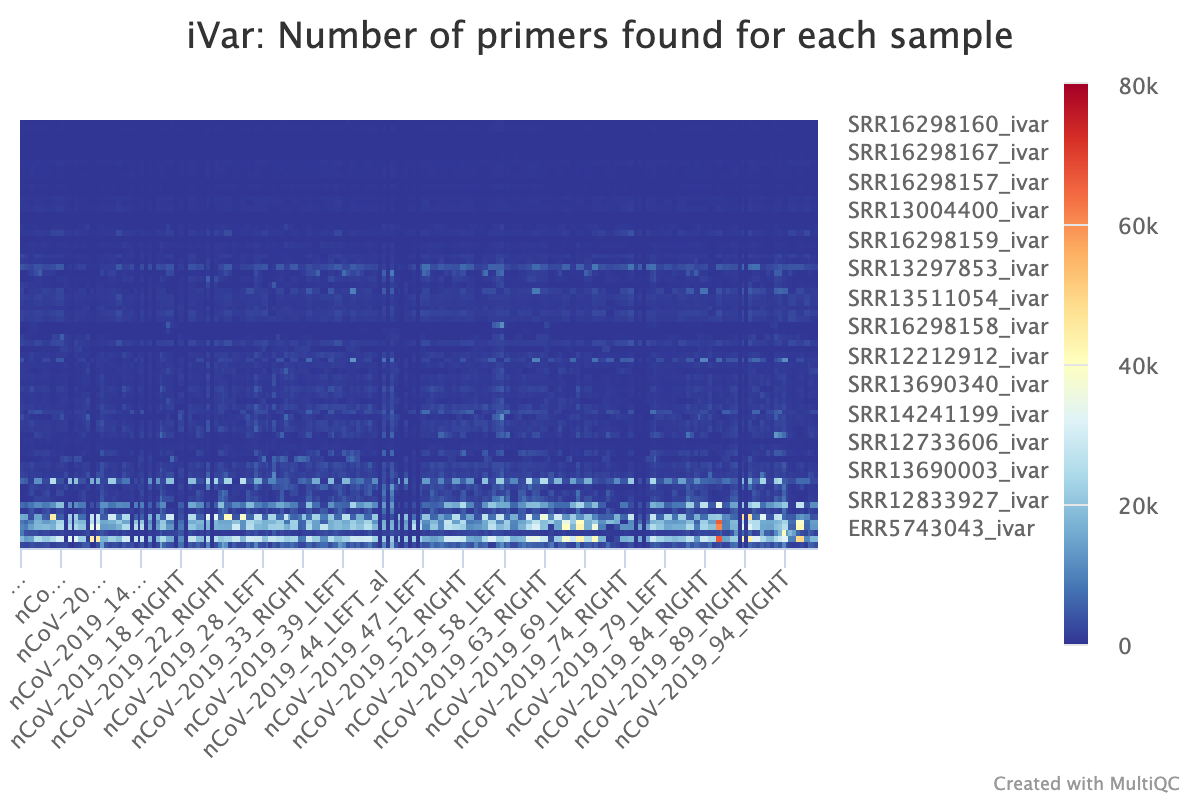
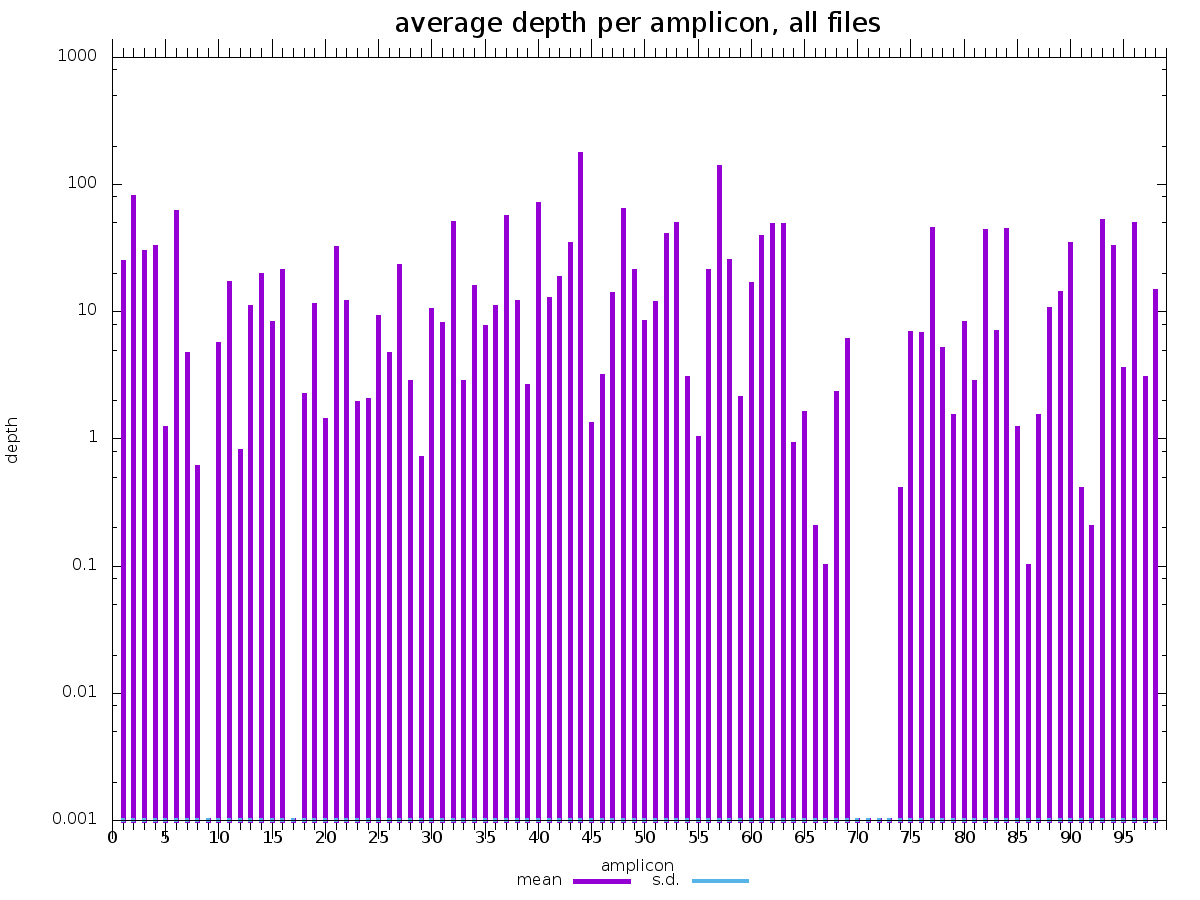
cecret/aci has two files : amplicon_depth.csv and amplicon_depth.png. There is a row for each sample in 'amplicon_depth.csv', and a column for each primer in the amplicon bedfile. The values contained within are reads that only map to the region specified in the amplicon bedfile and excludes reads that do not. A boxplot of these values is visualized in amplicon_depth.png.
cecret/samtools_ampliconstats has a file for each sample.
Row number 126 (FDEPTH) has a column for each amplicon (also without a header). To get this row for all of the samples, grep the keyword "FDEPTH" from each sample.
grep "^FDEPTH" cecret/samtools_ampliconstats/* > samtools_ampliconstats_all.tsv
There are corresponding images in cecret/samtools_plot_ampliconstats for each sample.
The primer bedfile is the file with the start and stop of each primer sequence.
$ head configs/artic_V3_nCoV-2019.primer.bed
MN908947.3 30 54 nCoV-2019_1_LEFT nCoV-2019_1 +
MN908947.3 385 410 nCoV-2019_1_RIGHT nCoV-2019_1 -
MN908947.3 320 342 nCoV-2019_2_LEFT nCoV-2019_2 +
MN908947.3 704 726 nCoV-2019_2_RIGHT nCoV-2019_2 -
MN908947.3 642 664 nCoV-2019_3_LEFT nCoV-2019_1 +
MN908947.3 1004 1028 nCoV-2019_3_RIGHT nCoV-2019_1 -
MN908947.3 943 965 nCoV-2019_4_LEFT nCoV-2019_2 +
MN908947.3 1312 1337 nCoV-2019_4_RIGHT nCoV-2019_2 -
MN908947.3 1242 1264 nCoV-2019_5_LEFT nCoV-2019_1 +
MN908947.3 1623 1651 nCoV-2019_5_RIGHT nCoV-2019_1 -
The amplicon bedfile is the file with the start and stop of each intended amplicon.
$ head configs/artic_V3_nCoV-2019.insert.bed <==
MN908947.3 54 385 1 1 +
MN908947.3 342 704 2 2 +
MN908947.3 664 1004 3 1 +
MN908947.3 965 1312 4 2 +
MN908947.3 1264 1623 5 1 +
MN908947.3 1595 1942 6 2 +
MN908947.3 1897 2242 7 1 +
MN908947.3 2205 2568 8 2 +
MN908947.3 2529 2880 9 1 +
MN908947.3 2850 3183 10 2 +
Due to the many varieties of primer bedfiles, it is best if the End User supplied this file for custom primer sequences.
First of all, this is a great thing! Let us know if tools specific for your organism should be added to this workflow. There are already options for 'mpx' and 'other' species.
In a config file, change the following relevant parameters:
params.reference_genome
params.primer_bed
params.amplicon_bed #or set params.aci = false
params.gff_file #or set params.ivar_variants = false
And set
params.species = 'other'
params.pangolin = false
params.freyja = false
params.nextclade = false #or adjust nexclade_prep_options from '--name sars-cov-2' to the name of the relevent dataset
params.vadr = false #or configure the vadr container appropriately and params.vadr_reference
Although not perfect, if 'params.filter = true', then only the reads that were mapped to the reference are returned. This should eliminate all human contamination (as long as human is not part of the supplied reference) and all "problematic" incidental findings.
This workflow has too many bells and whistles. I really only care about generating a consensus fasta. How do I get rid of all the extras?
Change the parameters in a config file and set most of them to false.
params.species = 'none'
params.fastqc = false
params.ivar_variants = false
params.samtools_stats = false
params.samtools_coverage = false
params.samtools_depth = false
params.samtools_flagstat = false
params.samtools_ampliconstats = false
params.samtools_plot_ampliconstats = false
params.aci = false
params.pangolin = false
params.freyja = false
params.nextclade = false
params.vadr = false
params.multiqc = false
And, yes, this means I added some bells and whistles so the End User could turn off the bells and whistles. /irony
No. Prior versions supported a tool called bamsnap, which had the potential to be great. Due to time constraints, this feature is no longer supported.
Never fear, they are still in nextflow's work directory if the End User really needs them. They are no longer included in publishDir because of size issues. The BAM files are still included in publishDir, and most analyses for SAM files can be done with BAM files.
Personally, we liked having stderr saved to a file because some of the tools using in this workflow print to stderr instead of stdout. We have found, however, that this puts all the error text into a file, which a lot of new-to-nextflow users had a hard time finding. It was easier to assist and troubleshoot with End Users when stderr was printed normally.
What is in the works to get added to 'Cecret'? (These are waiting for either versions to stabilize or a docker container to be available.)
- AWS compliant config file
- https://github.com/chrisruis/bammix
- https://github.com/lenaschimmel/sc2rf
- masking options for phylogenetic relatedness
Bedtools multicov was replaced by ACI due to processing times, but there are other processes that take longer.
Right now, the processes that take the most time are
- ivar trim
- freyja