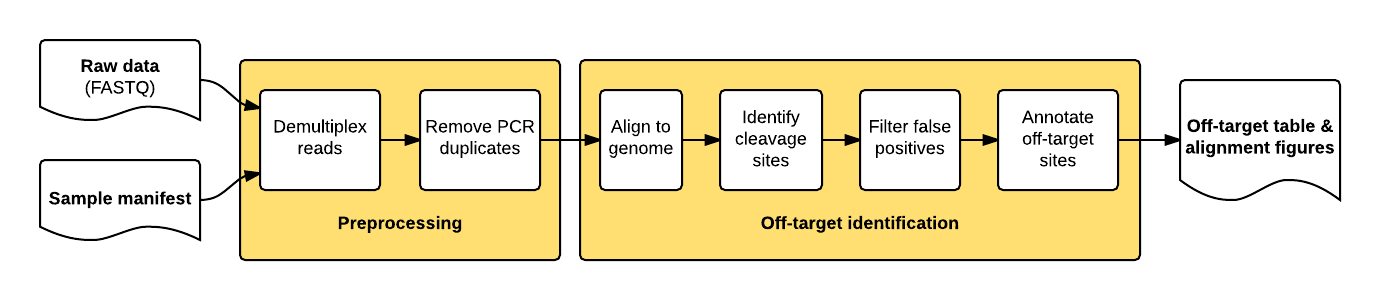
The guideseq package implements our data preprocessing and analysis pipeline for GUIDE-Seq data. It takes raw sequencing reads (FASTQ) and a parameter manifest file (.yaml) as input and produces a table of annotated off-target sites as output.
Tsai SQ, Zheng Z, Nguyen NT, Liebers M, Topkar VV, Thapar V, Wyvekens N, Khayter C, Iafrate AJ, Le LP, Aryee MJ, Joung JK. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol. 2015 Feb;33(2):187-197
Tsai SQ, Topkar VV, Joung JK, Aryee MJ. Open-source guideseq software for analysis of GUIDE-seq data. Nat Biotechnol. 2016 May 6;34(5):483
- Features
- Dependencies
- Getting Set Up
- Running the Full Analysis Pipeline
- Running Analysis Steps Individually
- Testing the guideseq Package
- Frequently Asked Questions
The package implements a pipeline consisting of a read preprocessing module followed by an off-target identification module. The preprocessing module takes raw reads (FASTQ) from a pooled multi-sample sequencing run as input. Reads are demultiplexed into sample-specific FASTQs and PCR duplicates are removed using unique molecular index (UMI) barcode information.
The individual pipeline steps are:
- Sample demultiplexing: A pooled multi-sample sequencing run is demultiplexed into sample-specific read files based on sample-specific dual-indexed barcodes
- PCR Duplicate Consolidation:Reads that share the same UMI and the same first six bases of genomic sequence are presumed to originate from the same pre-PCR molecule and are thus consolidated into a single consensus read to improve quantitative interpretation of GUIDE-Seq read counts.
- Read Alignment: The demultiplexed, consolidated paired end reads are aligned to a reference genome using the BWA-MEM algorithm with default parameters (Li. H, 2009).
- Candidate Site Identification: The start mapping positions of the read amplified with the tag-specific primer (second of pair) are tabulated on a genome-wide basis. Start mapping positions are consolidated using a 10-bp sliding window. Windows with reads mapping to both + and - strands, or to the same strand but amplified with both forward and reverse tag-specific primers, are flagged as sites of potential DSBs. 25 bp of reference sequence is retrieved on either side of the most frequently occuring start-mapping position in each flagged window. The retrieved sequence is aligned to the intended target sequence using a Smith-Waterman local-alignment algorithm.
- False positive filtering: Off-target cleavage sites with more than six mismatches to the intended target sequence, or that are present in background controls, are filtered out.
- Reporting: Identified off-targets, sorted by GUIDE-Seq read count are annotated in a final output table. The GUIDE-Seq read count is expected to scale approximately linearly with cleavage rates (Tsai et al., Nat Biotechnol. 2015).
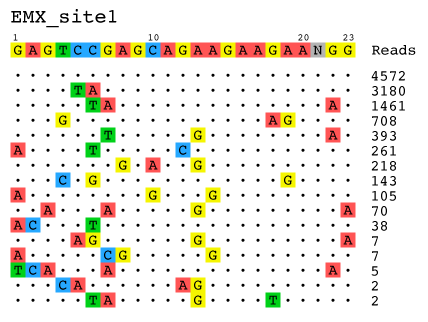
- Visualization: Alignment of detected off-target sites is visualized via a color-coded sequence grid, as seen below:
- Python (2.7)
- Reference genome fasta file (Example)
bwaalignment toolbedtoolsgenome arithmetic utility
To run guideseq, you must first install all necessary dependencies:
- Python 2.7: If a version does not come bundled with your operating system, we recommend the Anaconda scientific Python package.
- Burrows-Wheeler Aligner (bwa): You can either install bwa with a package manager (e.g.
brewon OSX orapt-geton Ubuntu/Debian), or you can download it from the project page and compile it from source. - Bedtools: You can either install bwa with a package manager (e.g.
breworapt-get), or you can download it from the project page and compile it from source.
For both bwa and bedtools, make sure you know the path to the respective executables, as they need to be specified in the pipeline manifest file.
The guideseq package requires a reference genome for read mapping. You can use any genome of your choosing, but for all of our testing and original GUIDE-seq analyses (Tsai et al. Nature Biotechnol 2015) we use hg19 (download). Be sure to (g)unzip the FASTA file before use if it is compressed.
Once all dependencies are installed, there are a few easy steps to download and set up the guideseq package:
- Obtain a copy of the guideseq package source code. You can either download and unzip the latest source from the github release page, or you use git to clone the repository by running
git clone --recursive https://github.com/aryeelab/guideseq.git - Install guideseq dependencies by entering the guideseq directory and running
pip install -r requirements.txt
Once all guideseq dependencies are installed, you will be ready to start using guideseq.
The guideseq package requires index reads from the MiSeq sequencing run for read consolidation. The default MiSeq Reporter settings do not generate index (I1, I2) reads. This feature can be enabled by adding the line
<add key="CreateFastqForIndexReads" value="1"> to the Miseq Reporter.exe.config file located in the Miseq Reporter installation folder. The default installation folder is C:\Illumina\MiSeqReporter. After modifying the config file it should look like this:
<appSettings>
... [LEAVE EXISTING LINES UNCHANGED] ...
<add key="CreateFastqForIndexReads" value="1"/>
</appSettings>The MiSeq Reporter service needs to be restarted for the change to take effect. Future runs of the GenerateFASTQ workflow (and probably other workflows) will generate I1 and I2 reads in addition to R1 and R2. All four of these reads files will be needed for guideseq analysis.
See page 29 of the Miseq Reporter User Guide for further instructions.
To run the full guideseq analysis pipeline, you must first create a manifest YAML file that describes all pipeline inputs. Once you have done so, you can simply run
python /path/to/guideseq.py all -m /path/to/manifest.yaml
to run the entire pipeline. Below are specific instructions detailing how to write the manifest file.
If you wish to run an example on our abridged test data, you can simply run
python guideseq/guideseq.py all -m test/test_manifest.yaml
from the guideseq root directory. The test_manifest assumes that both the bwa and bedtoolsexecutables are in your system PATH. You will see the pipeline results outputted to the test/output folder.
When running the end-to-end analysis functionality of the guideseq package, a number of inputs are required. To simplify the formatting of these inputs and to encourage reproducibility, these parameters are inputted into the pipeline via a manifest formatted as a YAML file. YAML files allow easy-to-read specification of key-value pairs. This allows us to easily specify our parameters. The following fields are required in the manifest:
reference_genome: The absolute path to the reference genome FASTA file.output_folder: The absolute path to the folder in which all pipeline outputs will be saved.bwa: The absolute path to thebwaexecutablebedtools: The absolute path to thebedtoolsexecutablewindow_size: Window size for search. Set to 10 for Cas9 and 75 for Cpf1.max_mismatches: The maximum number of mismatches allowed to report a sequence-matched off-targetundemultiplexed: The absolute paths to the undemultiplexed paired end sequencing files. The required parameters are:forward: The absolute path to the FASTQ file containing the forward reads.reverse: The absolute path to the FASTQ file containing the reverse reads.index1: The absolute path to the FASTQ file containing the forward index reads.index2: The absolute path to the FASTQ file containing the reverse index reads.
An example undemultiplexed field:
undemultiplexed:
forward: ../test/data/undemux.r1.fastq.gz
reverse: ../test/data/undemux.r2.fastq.gz
index1: ../test/data/undemux.i1.fastq.gz
index2: ../test/data/undemux.i2.fastq.gz
samples: A nested field containing the details of each sample. At least two samples must be specified: a "control" sample (to be used to filter out background off-target sites) and at least one treatment sample. The required parameters are:target: The sample targetsitesbarcode1: The forward barcodebarcode2: The reverse barcodedescription: A description of the sample
An example samples field:
samples:
control:
target:
barcode1: CTCTCTAC
barcode2: CTCTCTAT
description: Control
[SAMPLENAME]:
target: GAGTCCGAGCAGAAGAAGAANGG
barcode1: TAGGCATG
barcode2: TAGATCGC
description: EMX1
Below is an example of a full manifest file. Feel free to copy it and replace the parameters with your own experiment data. Remember that you can input more than just one treatment sample (e.g. the "EMX1" data below).
reference_genome: test/test_genome.fa
output_folder: test/output
bwa: bwa
bedtools: bedtools
demultiplex_min_reads: 1000
window_size: 75
max_mismatches: 7
undemultiplexed:
forward: test/data/undemultiplexed/undemux.r1.fastq.gz
reverse: test/data/undemultiplexed/undemux.r2.fastq.gz
index1: test/data/undemultiplexed/undemux.i1.fastq.gz
index2: test/data/undemultiplexed/undemux.i2.fastq.gz
samples:
control:
target:
barcode1: CTCTCTAC
barcode2: CTCTCTAT
description: Control
EMX1:
target: GAGTCCGAGCAGAAGAAGAANGG
barcode1: TAGGCATG
barcode2: TAGATCGC
description: EMX_site1
When running the full pipeline, the results of each step are outputted to the output_folder in a separate folder for each step. The output folders and their respective contents are as follows:
output_folder/demultiplexed: Contains the four undemultiplexed reads files (forward, reverse, index1, index2) for each sample.output_folder/umitagged: Contains the two umitgged reads files (forward, reverse) for each sample.output_folder/consolidated: Contains the two consolidated reads files (forward, reverse) for each sample.output_folder/aligned: Contains an alignment.samfile for each sample.output_folder/identified: Contains a tab-delimited.txtfile for each sample with an identified off-target in each row.output_folder/filtered: Contains a tab-delimited.txtfile for each sample containing the identified DSBs that are background sites (not off-targets)output_folder/visualization: Contains a.svgvector image representing an alignment of all detected off-targets to the targetsite for each sample.
The final detected off-target sites are placed in the output_folder/identified folder, with one .txt file for each sample specified in the manifest. The fields that are populated in each row of these off-target files are specified below:
####Output Off-Targets .txt Fields:
BED Chromosome: Window chromosomeBED Min.Position: Window 0-based start positionBED Max.Position: Window 0-based end positionBED Name: Name of windowFilename: The name of the current.SAMfile used in analysis.WindowIndex: Index number of windowChromosome: Chromosome corresponding to position with maximum reads in window (matchesBED Chromosome)Position: Position with maximum number of reads in windowSequence: The window sequence, starting 25 bp upstream and ending 25 bp downstream ofChromosome:Position+.mi: Number of forward reads with distinct molecular indices-.mi: Number of reverse reads with distinct molecular indicesbi.sum.mi: Sum of the+.miand-.mifields (GUIDE-seq Read Count)bi.geometric_mean.mi: Geometric mean of the+.miand-.mifields+.total: Total number of forward mapping reads-.total: Total number of reverse mapping readstotal.sum: Sum of+.totaland-.totalfieldstotal.geometric_mean: Geometric mean of the+.totaland-.totalfieldsprimer1.mi: Number of reads amplified by forward primer with distinct molecular indicesprimer2.mi: Number of reads amplified by reverse primer with distinct molecular indicesprimer.geometric_mean: Geometric mean of theprimer1.miandprimer2.mifieldsposition.stdev: Standard deviation of positions within genomic windowOff-Target Sequence: Off-target sequence derived from genome referenceMismatches: Number of mismatches between the intended target sequence and the off-target sequenceLength: Length of the target sequenceBED off-target Chromosome: Off-target chromosomeBED off-target start: Off-target 0-based start positionBED off-target end: Off-target 0-based end positionBED off-target name: Off-target nameBED Score: Field to conform to standard BED formatStrand: Indicates the strand of detected off-target site.+for forward strand and-for reverse strandCells: Cell typeTarget site: Targetsite nameTarget Sequence: Intended target site sequence (including PAM)
The key fields for interpreting this output and identifying off-target sites are: BED off-target Chromosome, BED off-target start, BED off-target end, BED off-target name, BED off-target strand, Off-Target Sequence, bi.sum.mi
The outputted visualizations are in the .svg vector format, which is an open image standard that can be viewed in any modern web browser (e.g. Google Chrome, Apple Safari, Mozilla Firefox), and can be viewed and edited in any vector editing application (e.g. Adobe Illustrator). Because the output visualizations are vector images, they can be scaled up or down infinitely without a loss in quality, and can also be edited as shapes with ease. This makes the images produced by the guideseq package ideal for posters, presentations, and papers.
In addition to end-to-end pipeline analysis functionality, the guideseq package also allows for every step fo the analysis to be run individually. Here we have detailed the required inputs and expected outputs of each step. For each step, we have included a "runnable example" command that can be executed from the guideseq root directory to run that step on the included sample data. These "runnable example" snippets put their output in the test/output folder.
- Functionality: Given undemultiplexed sequence files and sample barcodes specified in the manifest, output the demultiplexed sample-specific reads in FASTQ format. The forward, reverse, and two index files for each sample in the manifest are outputted to the
output_folder/consolidatedfolder. - Required Parameters:
-m or --manifest: Specify the path to the manifest YAML file
- Runnable Example:
python guideseq/guideseq.py demultiplex -m test/test_manifest.yaml
-
Functionality: Given the demultiplexed files in the folder
output_folder/undemultiplexed(whereoutput_folderis specified in the manifest), 'tag' the reads by adding the UMI barcode sequence to the FASTQ read name header in preparation for the subsequent PCR duplicate read consolidation step. The forward and reverse files for each sample in the manifest are outputted to theoutput_folder/umitaggedfolder. -
Required Parameters:
--read1: Path to the forward demultiplexed reads file (FASTQ)--read2: Path to the reverse demultiplexed reads file (FASTQ)--index1: Path to the index1 demultiplexed reads file (FASTQ)--index2: Path to the index2 demultiplexed reads file (FASTQ)--outfolder: Path to the folder in which the output files will be saved
-
Runnable Example:
python guideseq/guideseq.py umitag --read1 test/data/demultiplexed/EMX1.r1.fastq \ --read2 test/data/demultiplexed/EMX1.r2.fastq \ --index1 test/data/demultiplexed/EMX1.i1.fastq \ --index2 test/data/demultiplexed/EMX1.i2.fastq \ --outfolder test/output/
-
Functionality: Given undemultiplexed sequence files and sample barcodes specified in the manifest, output the consolidated forward and reversed reads to the
outfolder. -
Required Parameters:
--read1: Path to the forward umitagged reads file (FASTQ)--read2: Path to the reverse umitagged reads file (FASTQ)--outfolder: Path to the folder in which the output files will be saved
-
Optional Parameters:
--min_quality: The minimum quality of a read for it to be considered in the consolidation--min_frequency: The minimum frequency of a read for the position to be consolidated
-
Runnable Example:
python guideseq/guideseq.py consolidate --read1 test/data/umitagged/EMX1.r1.umitagged.fastq \ --read2 test/data/umitagged/EMX1.r2.umitagged.fastq \ --outfolder test/output/
-
Functionality: Given the consolidated forward and reverse reads, execute a paired-end mapping of the sequences to the reference genome using the
bwapackage. Outputs an alignment.samfile to theoutfolder. -
Required Parameters:
--bwa: Path to thebwaexecutable--genome: Path to the reference genome FASTA file--read1: Path to the consolidated forward read FASTQ file--read2: Path to the consolidated reverse read FASTQ file--outfolder: Path to the folder in which the output files will be saved
-
Runnable Example:
python guideseq/guideseq.py align --bwa bwa --genome test/test_genome.fa\ --read1 test/data/consolidated/EMX1.r1.consolidated.fastq\ --read2 test/data/consolidated/EMX1.r2.consolidated.fastq\ --outfolder test/output/
-
Functionality: Given the alignment samfile for a given site, a reference genome, and a target sequence, output a tab-delimited
.txtfile containing the identified off-target sites. -
Required Parameters:
--aligned: Path to the site-specific alignment.samfile.--genome: Path to the reference genome FASTA file.--outfolder: Path to the folder in which the output files will be saved.--target_sequence: The sequence targeted in the sample (blank for control sample)
-
Optional Parameters:
--description: Specify additional information about the sample.
-
Runnable Example:
python guideseq/guideseq.py identify --aligned test/data/aligned/EMX1.sam\ --genome test/test_genome.fa --outfolder test/output/\ --target_sequence GAGTCCGAGCAGAAGAAGAANGG --description EMX1
-
Functionality: Given the identified site
.txtfiles for a treatment and control samples, output a.txtfile in the same format as outputted by theidentifystep containing the sites filtered out as false-positives. -
Required Parameters:
--bedtools: Path to thebedtoolsexecutable--identified: Path to the.txtfile outputted by theidentifystep for a treatment sample.--background: Path to the.txtfile outputted by theidentifystep for a control sample.--outfolder: Path to the folder in which the output files will be saved.
-
Runnable Example:
python guideseq/guideseq.py filter --bedtools bedtools\ --identified test/data/identified/EMX1_identifiedOfftargets.txt\ --background test/data/identified/control_identifiedOfftargets.txt\ --outfolder test/output/
-
Functionality: Given an identified off-target sites
.txtfile, output an alignment visualization of the off-target sites. -
Required Parameters:
--infile: Path to the input.txt.off-targets file--outfolder: Path to the outputted folder containing the outputted.svggraphic
-
Optional Parameters:
--title: Specify the title of the visualization, to be printed at the top of the graphic. Useful for posters and presentations.
-
Runnable Example:
python guideseq/guideseq.py visualize --infile test/data/identified/EMX1_identifiedOfftargets.txt\ --outfolder test/output/ --title EMX1
In the spirit of Test-Driven Development, we have written end-to-end tests for each step of the pipeline. These can be used to ensure that the software is running with expected functionality.
NOTE: Due to differences in sorting between different versions of the bwa package, you must be using bwa v0.7.9a for these tests to work. We also recommend that you use bedtools v2.25.0 when running these tests for consistency's sake.
For ongoing testing and development, we have created an abridged set of input data and expected output data for each step of the pipeline. This way, changes to the pipeline can be quickly tested for feature regression.
To run these tests, you must first install the nose testing Python package.
pip install nose
Then, from the guideseq root directory, simply run
nosetests
and the regression tests for each pipeline step will be run.
If you have more time, we have prepared a bash script that downloads and compiles all dependencies from source, downloads a fresh reference genome and a full GUIDE-seq sequencing data run, and performs a full test of the entire pipeline. This test takes a long time, but we require that it be run before we tag a new release.
To run the full large test, enter the guideseq/test folder and run
./large_test.sh
Then, sit back and watch the full large testing process unfold automatically in the terminal.
If you wish to run a full GUIDE-Seq dataset through the analysis pipeline, you may find it and a test manifest (to be altered depending on your dependency locations) here:
http://aryee.mgh.harvard.edu/guideseq/data/guideseq_test_fastq.zip
which should be used with the following reference genome:
http://www.broadinstitute.org/ftp/pub/seq/references/Homo_sapiens_assembly19.fasta
If you already have demultiplexed data, you can run the pipeline on the data by running each step after demultiplexing individually, as described in the "Running Analysis Steps Individually" section above. Be sure to run the individual steps in the following orders:
umitagconsolidatealignidentifyfiltervisualize
Yes. If your reads do not have UMIs, you can run the pipeline on previously demultiplexed data as described in the "Running Analysis Steps Individually" section above, starting with the align step. Note that we have not analyzed such data ourselves! We suspect that PCR duplication bias may affect the quantitative interpretion of GUIDE-Seq read counts, but have not explored this.