CATHI is now also available for remote reciprocal BLAST searches, without the need for downloading BLAST databases. You just need to provide the backward BLAST genome, which is one protein FASTA file.
There might be some installation issues, if you need help to set up Docker and CATHI: write a mail to [email protected] and we can arrange a meeting. CATHI is still under development, any help is useful, so don't hesitate to write a mail.
Thanks!
Symmetrical BLAST and target sequence search web interface with Django, Gunicorn, Ngninx, PostgreSQL, Celery, RabbitMQ, E-Direct, BLAST, Snakemake and Miniconda.
CATHI is a user-friendly bioinformatics tool that performs reciprocal BLAST searches, generates multiple sequence alignments, and builds phylogenetic trees. It is integrated with the workflow management system ``Snakemake`, providing a streamlined and efficient way to manage the entire bioinformatics pipeline. Furthermore, CATHI offers additional features that make it a powerful and flexible solution for researchers across various fields. CATHI generates interactive plots and tables in standalone HTML documents, which enables users to visualize and analyze complex biological data easily. CATHI integrates the NCBI's EDirect tool, which allows for sequence and paper searches directly within the web interface. It also enables users to download taxonomic-specific protein sequences based on the results of their EDirect searches.
In addition, CATHI includes tools for managing local protein BLAST databases. Researchers can download and format custom databases, providing greater control and flexibility over the data used in their analyses. CATHI also includes a refined alignment and phylogenetic tree reconstruction process that leverages the protein domains of the CDD database using the RPS-BLAST tool.
CATHI is highly sophisticated and powerful, offering a range of features and capabilities ideal for target sequence searches. It enables performing reciprocal and one-way BLAST searches within local and remote databases, providing valuable insights into the evolutionary relationships and functional characteristics of sequences. CATHI's integration into a docker container network streamlines its installation process.
The only requirement for installation is a local Docker-Desktop installation, which you can download under the following link: docker-desktop. Make sure to create an account for Docker and to be logged into Docker before you install CATHI.
You may also need to configure Docker. Thus, it may require a lot of your RAM and processors. You can do this by adding
a .wslconfig file in your User directory. For more information I recommend reading this article.
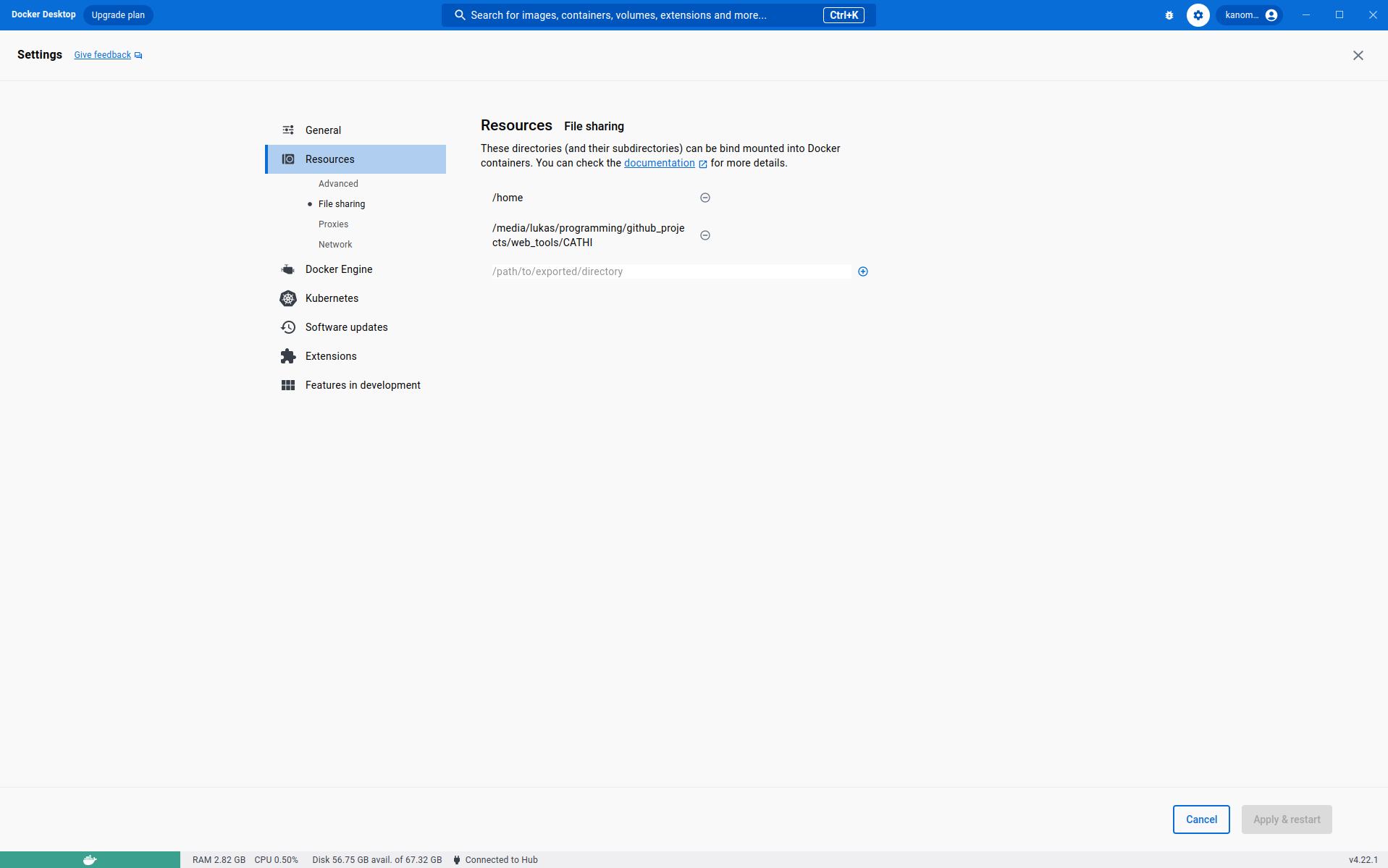
If you are working on a UNIX machine, you may need to enable file sharing. Therefore, open your docker-desktop application and switch to the Settings tab. Open the settings page and add the path to CATHI on the File Sharing tab in the Resources section (see following image).
Download and decompress this repository via the Download ZIP button of the <> Code tab or with git clone [email protected]:Kanomble/celery_blast.git.
Open a terminal and use the cd command to point to the local version of the CATHI project repository (e.g., if you have downloaded and decompressed CATHI
in the directory: C:\Users\Test\Documents\CATHI, open your powershell and execute the command cd "C:\Users\Test\Documents\CATHI".
Use the ls command to confirm that your terminal/powershell points to the correct directory, the output of ls should include following files and directories:
celery_blast data docker-compose-production.yml docker-compose.yml Dockerfile LICENSE nginx README.md requirements.txt tmp.
mkdir CATHI
cd CATHI
git clone [email protected]:Kanomble/celery_blast.git
cd celery_blast
docker compose -f docker-compose-production.yml upThe application can get installed by submitting the command: docker-compose up for the development environment or by:
docker-compose -f docker-compose-production.yml up for the production environment.
Once installed, CATHI is accessible via: http://127.0.0.1:1337/blast_project/ for the production environment
and via: http://127.0.0.1:8080/blast_project/ for the Django development server.
Once you have installed CATHI you should see, that the containers within the Docker-Desktop application are listed green. Here is an example for the production environment:
Next, you need to perform a last Set-Up step, therefore you need to download the Conserved-Domain-Database and the RefSeq and GenBank assembly summary files. This can be done by clicking on the Start CATHI Tool Set-Up Procedure button, that appears on the home dashboard after registering and logging into CATHI.
The docker client will pull remotely available images, including the base image for this application,
an image for the PostgreSQL database and finally an image for the RabbitMQ message broker.
Docker images are pulled from this DockerHub.
It is recommended to install the application with the remotely available images and the docker compose up command.
It is possible to build the necessary images from the Dockerfile of this repository. You can change and adapt the dockerfile according to the software
tools you need, keep in mind to adapt the docker-compose.yml or the docker-compose-production.yml files if you want to use local Docker images of this tool.
Docker creates seven containers named: celery_blast_X_1 where X is a synonym for
nginx, worker, flower, web, postgres and rabbitmq.
CATHI is a server site tool, by starting the container network, your local computer will be used as a web
server. Django is the underlying web-framework and gunicorn serves as the WSGI HTTP Server. Both applications reside
in the CATHI base image. Nginx is used as a reverse proxy server, it directs client requests to gunicorn.
The long-running background tasks are managed by rabbitmq and celery, thus triggered processes are picked up by
the message broker rabbitmq and passed to a queue, if a celery-worker is free, the process is executed. The task progress
is saved within the postgresql database within the django_celery_results_taskresult table, which enables task monitoring.
The flower container can be used to monitor the celery-worker. The reciprocal BLAST pipeline and the normal
one-way BLAST pipelines are integrated into a Snakefile, which is used by the workflow management system snakemake.
Customization of Snakefiles enables user defined post-processing. In addtion, a jupyter-notebook container is
integrated into the CATHI container network. Configuration is done within the .env.prod file.
All important environment variables are defined within this file
(e.g. the DJANGO_ALLOWED_HOSTS and the SECRET_KEY variables).
To execute the integrated reciprocal BLAST pipeline of CATHI, certain data must be set up by the user. This includes the query sequences from a particular organism/genome file, a forward BLAST database that will serve as the search space, a backward BLAST database, the scientific name of the organism from which the query sequences were obtained, and a project title. Additionally, the user can modify some BLAST settings, such as the number of output sequences per query sequence (num_alignments) or the e-value cut-off. The BLAST databases can be selected from a special drop-down menu.
The forward BLAST database acts as a search space in which putative orthologous sequences can be located, while the backward BLAST database must contain the genome file from which the query sequences were obtained. Prior to saving a project into the database or executing the pipeline, the user-provided data undergoes validation to ensure that it meets the necessary criteria. If any of the validations fail, accurate error messages are displayed within the relevant form fields to ensure a smooth pipeline execution.
The pipeline comprises the following steps:
- Forward BLAST (default BLAST settings: e-value=0.001, word-size=3, threads=1, num_alignments=10000, max_hsps=500)
- Backward BLAST preparation (extracting homologous target sequences of the forward BLAST)
- Backward BLAST (BLAST search of the homologous target sequences against the genome of the query sequences)
- Extracting Reciprocal Best Hits (RBHs, this is done via pandas merging tools)
- Post-processing of RBHs (inference of taxonomic information, statistics, HTML and CSV tables, basic result plots)
- Extraction of RBH-sequences separated by query sequences
- Multiple sequence alignment of each set of RBHs with MAFFT
- Phylogenetic inference of each set of RBHs with FastTree
- Post-processing of the phylogenetic tree with ete3
- CDD domain search of target sequences
Further CATHI post-processing procedures outside the scope of this pipeline involves:
- Combining taxonomic information of the underlying database with the RBH result table
- Building an interactive bokeh plot, that enables intuitive result interpretation
- Filter RBHs based on taxonomy, e-value, bitscore, sequence length and percent identity
- Download a selection of protein identifier
- Refined phylogenetic inference with CDD domains of the RBHs
- Conducting RPS-BLAST with a specified set of RBHs
- Conducting a principal component analysis (PCA) based on the percent identity of the inferred domains with respect to the query sequence domains
- Building an interactive bokeh plot with the first two principal components and the taxonomic information within the RBH result table
- Refine the phylogeny by slecting RBHs directly from the PCA bokeh-plot to conduct a conserved-domain corrected phylogenetic inference
- Synteny analysis
- Select up to ten different RBHs for the inference of syntenic regions with the clinker tool
- The clinker result is an interactive HTML document that can be changed according to user needs
Further CATHI target-sequence search features:
- Target sequence search with NCBIs Entrez-Direct tool
 The X and Y axes of the scatter plot are parameters of the dataset, such as bitscore, e-value, sequence identity (pident), and sequence lengths, enabling the
exploration of relationships among these RBHs. The interactivity of the scatter plot is facilitated by Bokeh, a powerful
visualization library. Users can dynamically manipulate the dataset through filtering options informed by the
taxonomic information associated with each RBH. In addition, Bokeh provides a lasso tool to select specific RBHs
within the graph. The lasso tool can be selected from the Bokeh tool-panel displayed at the right side of the figure.
This interactive feature empowers researchers to dissect real-time taxonomic trends and relationships among RBHs,
unveiling underlying patterns and insights.
The X and Y axes of the scatter plot are parameters of the dataset, such as bitscore, e-value, sequence identity (pident), and sequence lengths, enabling the
exploration of relationships among these RBHs. The interactivity of the scatter plot is facilitated by Bokeh, a powerful
visualization library. Users can dynamically manipulate the dataset through filtering options informed by the
taxonomic information associated with each RBH. In addition, Bokeh provides a lasso tool to select specific RBHs
within the graph. The lasso tool can be selected from the Bokeh tool-panel displayed at the right side of the figure.
This interactive feature empowers researchers to dissect real-time taxonomic trends and relationships among RBHs,
unveiling underlying patterns and insights.
Use appropriate BLAST databases. If you want to search in more complete genomes, create a database that contains genome sequences
with a completeness level of Chromosome or Complete Genome. The e-value is more accurate for bigger databases, adjust the
e-value according to your needs, this may have a huge effect on your inferred RBHs. Adjust the num_alignments parameter if
you work with huge databases, especially if you are working with more common sequences.
The backward BLAST database should contain only one genome that corresponds to the taxonomic unit translated provided scientific name.
First, you need to tell CATHI to download the refseq or genbank assembly summary file from the refseq FTP or genbank FTP directories. The application loads the processed entries of the summary file into a pandas dataframe, that is displayed in the BLAST database transaction dashboard after pressing the submit button. Possible BLAST database user specifications are the level of assembly completeness (e.g. 'Complete Genome', 'Chromosome', 'Contig' and 'Scaffold') and (multiple) taxonomic information (e.g. 'Cnidaria, Mammalia').
E.g. if you want to create a high quality database containing only species from the phylum Cyanobacteriota, you have to specify
this during database creation. This can be done by typing Cyanobacteriota into the field "Scientific Names (sep. by ",")" and by
checking the assembly levels Complete Genome and Chromosome.
If the user submits the form, a BlastDatabase model instance and a
database directory, with a csv file containing the database table, is created.
The model is saved into the database, the database is not downloaded and formatted directly.
The download and formatting procedure has to be started separately, which enables the user to validate database entries.
The download and format process progression is visualized on the database dashboard.
Available databases are shared between users.
The second option to obtain BLAST databases is to upload your own genomes. Currently, only genomes with protein sequences are supported. There are two different forms that can be used for uploading your own genome files.
- The first form allows uploading a concatenated genome FASTA files with metadata file fields such as a taxmap file, which holds taxonomic information, an organism file, an assembly level file and an assembly accession file. Most of these files are not mandatory.
- The other form allows uploading of multiple, single genome files together with their valid scientific organism names.
All necessary software packages are deployed within docker container. Those containers are wrapped into a network using docker-compose.
Django, Gunicorn, and Nginx are commonly used technologies for building and deploying web applications. In a Docker network, these technologies can be used together to create a scalable and efficient web application stack. Django is a popular Python-based web framework used for building web applications. Gunicorn is a Python WSGI HTTP server that can serve Django applications. Nginx is a high-performance web server that can act as a reverse proxy, load balancer, and serve static files. When using Django, Gunicorn, and Nginx in a Docker network, each technology can be run in a separate Docker container. The Django application can be run using Gunicorn as a WSGI HTTP server, while Nginx can be used as a reverse proxy to direct traffic to the appropriate container. This setup can be scaled horizontally by adding more containers to handle increased traffic. In addition, Docker's networking features can be used to ensure that traffic is properly directed to the appropriate container. Overall, using Django, Gunicorn, and Nginx in a Docker network can create a highly scalable and efficient web application stack.
The open source tool celery is used to integrate a task queuing system within the django container. All background tasks are
wrapped into celery tasks. Celery enables task distribution across threads which enables the simultaneous processing of various reciprocal or one-way BLAST
projects. The celery worker process is triggered in the celery_worker container, tasks can be monitored via dedicated web-interfaces or within this
container. Accurate log-messages are displayed within the terminal window of the celery_worker container. Celery tasks are saved
in the PostgreSQL database by using the TaskResult model instance of the django_celery_results django application
(installed via the python package django-celery-results). Detailed information about Celery can be found on the official
project documenation page.
In order to enable reproducibility and an easy-to-use workflow execution, the workflow engine snakemake is used.
Snakemake associated snakefiles reside in a static directory celery_blast/celery_blast/static/snakefiles.
They can be used outside this application, e.g. if the researcher needs to use additional settings or want to implement own post-processing procedures.
Different snakefiles are designed to execute the desired workflows.
Currently, there are Snakefiles for the One-Way BLAST remote and local searches and the reciprocal BLAST analysis.
Execution of snakemake is wrapped in functions within the tasks.py files,
which are decorated with the celery @shared_task decorator. Those functions are queued up by rabbitmq and are processed by the celery worker.
Snakemake is executed with the subprocess.Popen interface which spawns a child process for every Snakemake workflow.
Snakemake's working directory is the current project directory, e.g. if you executed the snakemake pipeline for a reciprocal BLAST
project, the CWD of snakemake is the path that points to this project. Default project paths are defined in the celery_blast/settings.py file.
Detailed information about Snakemake can be found on the project documentation page.
Django models reside inside project specific models.py files. Models are translated to database tables.
Documentation about the django.db.models package can be found here.
Relationships between models are managed with django model functions.
The models.ForeignKey() function is used for OneToMany / ManyToOne relations.
Additionally, there are the models.OneToOneField() and models.ManyToManyField() functions.
Relationships can get further described with related_name and related_query_name parameters, described in
this Django documentation section.
Model managers reside inside project specific managers.py files.
Manager classes are responsible for the initial creation of the database models, such as create_blast_project(fields=values ...).
Those functions can be used to trigger side effects during initialization of the database entry.
E.g. creation of blast project directories or specific setting files, such as the snakemake configuration file.
When the user clicks the download button, a celery asynchronous task is initiated.
This task consists of multiple subtasks, each responsible for performing specific steps in the database creation process.
Celery allows for task monitoring through a logger and the TaskResult model of the result backend.
The task is triggered by a function called download_blast_databases_based_on_summary_file
in the refseq_transactions/tasks.py file, which has three main steps. Firstly, it attempts to download and decompress
all files stored in a table created during BlastDatabase model creation (as described in the above section).
The celery task then formats the downloaded protein fasta files into BLAST databases and generates
an alias file similar to the one created with the blastdb_aliastool.
All three main steps are performed using the subprocess.Popen interface.
In the first step, the Unix commands wget and gzip are used to download and simultaneously
decompress genome assemblies from the NCBI FTP server. This is accomplished with the following command:
wget -qO- ftp_path | gzip -d > assembly_file.faa.
If this command produces an error (returncode != 0), it is retried up to a maximum of ten times.
If the command still fails after ten attempts, the assembly file is skipped and removed from the csv table,
the FTP path pointing to the file is written to a logfile.
Finally, in the next step, makeblastdb is used to format the downloaded and decompressed assembly files into BLAST databases.
The makeblastdb module is a useful tool for building custom BLAST databases.
By default, it creates a database based on the input sequences.
For example, if you submit the following command: makeblastdb -in .\prot_1_db.faa -dbtype prot -taxid 1140 -blastdb_version 5,
a database named bw_prot_db.faa will be created.
The -taxid parameter is used to assign the taxonomic node 1140 (corresponding to Synechococcus elongatus 7492)
to all sequences in the bw_prot_db.faa fasta file.
If you need to format multiple fasta files using the makeblastdb program, there are two options available.
First, you can provide multiple fasta files to the -in parameter.
Alternatively, after formatting each fasta file individually, you can create a .pal database alias file that lists
all existing databases. The blastdb_aliastool program can also be used to create this alias file for you.
If the sequences in the fasta files have the same taxonomic node, you can use the -taxid parameter to assign the taxid to the program.
However, if they have different taxonomic nodes, you should use the -taxid_map parameter instead.
makeblastdb -in .\prot_1_db.faa -dbtype prot -taxid 1140 -blastdb_version 5
makeblastdb -in .\prot_2_db.faa -dbtype prot -taxid 1844971 -blastdb_version 5
blastdb_aliastool -dblist 'prot_1_db.faa prot_2_db.faa' -dbtype prot -title combined_db -out combined_db
blastp -query .\test.faa -db combined_db -out blast_out.table -outfmt "6 qseqid sseqid evalue bitscore qgi sgi sacc pident nident mismatch gaps qcovhsp staxids sscinames scomnames sskingdoms stitle"BLASTP -outfmt 6 output formats are described here.
The third step creates an alias file for all BLAST databases that have been formatted in the previous step.
The alias file is the file, that is created by the blastdb_aliastool.
Example of the combined_db.pal file:
#
# Alias file created 04/17/2021 12:50:29
#
TITLE combined_db
DBLIST "prot_1_db.faa" "prot_2_db.faa"
The .pal file combines different formatted BLAST databases so that they can be used as one combined database.
This is useful for databases with duplicate sequences, they normally have an identifier (accession number) that starts with WP.
During execution the underlying database (e.g. BlastDatabase or BlastProject) model OneToOne field gets updated with the appropriate celery TaskResult model.
This allows interaction with the associated celery task and can be used for displaying the progress of the task.
- fix error in domain corrected phylogeny
- the target sequence may not be part of the dataframe for the construction of phylogenies
- add ajax calls to CDD search ...
- add capitalize or something similar to name validations
- fix correct error displaying if there are no results in a reciprocal BLAST search
- add warning during project creation if in the database reside more than one organism with the specified taxid
- fix evalue in axis changing within bokeh plots
- refactor calculation of database normalized tables
- seems to be zero in some cases (which is wrong)
- add validation for entrez_queries
- add videos, links to init page
- add setup option after installation -> directly download CDD/refseq/genbank assembly summary files (maybe add a wizard)
- add error message if filtered database table is empty
- Interaction with NCBI (Entrez) via python Biopython package
- Celery Project Documentation
- Documentation for snakemake
- Documentation for celery-progress - youtube tutorial celery-progress
- BLAST DB FTP server description
- Documentation for the E-Direct tool