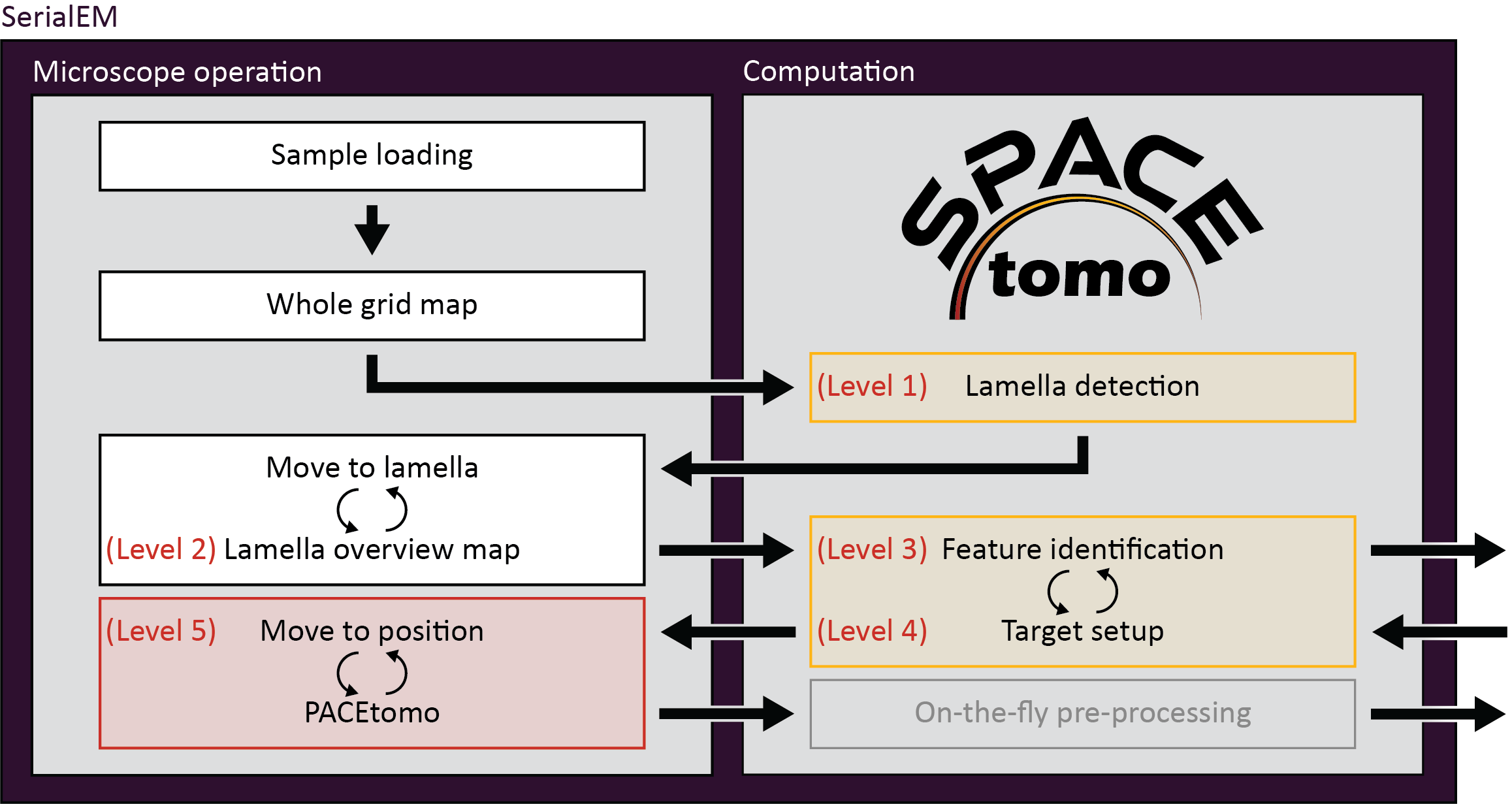
Smart Parallel Automated Cryo Electron tomography (SPACEtomo) is a set of SerialEM and Python scripts to fully automate the cryoET data collection workflow. Please refer to the publication for more details.
For now, the target selection model has been only trained on Yeast data. You can still use SPACEtomo for automated lamella detection and montage collection as well as setting up a dense target pattern covering the whole lamella avoiding ice chunks and cracks.
I plan on training models for a variety of organisms in the future with the help of the community.
- Hardware
- Requirements
- Installation
- Usage
- Video tutorials
- Troubleshooting
- Recent changes
- Training Data
- Future plans
Edit_Higor: This version of SPACEtomo has been implemented on a Thermo Scientic Krios G4 equipped with a Falcon 4i direct electron detector. In addition, we also use a SupportPC (with GPU and internet connection) where we mount the Microscope storage server to have access to the acquired maps.
SPACEtomo has only been tested on a Thermo Scientic Krios equipped with a Gatan K3 direct electron detector at this point although it should also work on other Thermo Scientific intstruments. A recent version of the open-source microscope control software SerialEM should be installed and calibrated. It also requires the PACEtomo scripts.
SPACEtomo does not require the installation of any stand-alone software. However, it does require SerialEM 4.1 or higher capable of running Python scripts.
You can run the following lines of code in a SerialEM script window to test if Python is configured correctly:
#!Python
import serialem as sem
sem.OKBox("Python works!")If you get an error message, please consult the SerialEM website on how to setup Python for SerialEM.
Additionally, you will require several Python modules. I highly recommend using the miniconda package manager (you will need network connection).
For our setup, I dowloaded and installed Anaconda on the SupportPC running Windows (because SerialEM runs also in Windows). Then I open the Anaconda launcher and using the Anaconda Powershell I've installed the dependencies as following:
If everything goes well, you can install all dependencies by downloading the SPACEenvironment.yaml file and running the following line the Anaconda Prompt:
conda env create -f <your path>\SPACEenvironment.yaml
This may take 10-20+ minutes to collect all the packages. If it fails or you are impatient you can install the packages manually step by step.
After this you can continue with setting the Python path (in the SerialEM properties file) to this PyTorch environment you have just created.
Details
Create a new environment using Python 3.9:
conda create -n SerialEM_pytorch python=3.9
conda activate SerialEM_pytorch
The next step is to install the deep learning framework PyTorch. This should be as simple as using the following command but please double check the PyTorch installation instructions for your system!
conda install pytorch torchvision pytorch-cuda=11.8 -c pytorch -c nvidia
Let's verify the installaion and check if PyTorch has access to your GPU.
From the command line type:
python
Then enter:
import torch
torch.cuda.is_available()If the output is not True, you have some troubleshooting to do. In my case, for example, I could fix it by updating the nvidia driver on our K3 computer. Here is some info on PyToch and Cuda compatibility and here some info on Cuda and driver compatibility. Good luck!
The next step is to install the segmentation model nnU-Net:
pip install nnunetv2
If you run into issues, here are their installation instructions.
Finally, we need YOLOv8 for the lamella detection model:
pip install ultralytics
With that, all dependencies should be ready to go!
To let SerialEM access this environment, we have to adjust the SerialEM_properties.txt file to include the Python path to our environment:
PathToPython 3.9 <your path>\envs\SerialEM_pytorch
You can find the SerialEM properties file in the ProgramData\SerialEM\ folder, and the path to our SerialEM_pytorch file, using the command conda env list (in the Anaconda Powershell) and copying the respective path of the environment.
To install SPACEtomo, simply download and unpack the repository on the PC running SerialEM, and make note of the path to the folder. This folder contains all scripts but still requires the deep learning models. Download the lamella detection model and the lamella segmentation model (Yeast) and extract them into the SPACEtomo folder. You should now have a XXX_yolo8.pt file and a model folder with 5 subfolders in your SPACEtomo folder.
Copy the SPACEtomo.py script into a SerialEM script window and adjust the SPACE_DIR setting to the path of the SPACEtomo folder.
SPACE_DIR = "X:\\<your path>\\SPACEtomo"Copy the SPACEtomo_postAction.py script into another SerialEM script window.
You also need the PACEtomo scripts (v1.7+) ready and working inside SerialEM.
The usage instructions for SPACEtomo assume that PACEtomo is already able to run normally on your setup.
SPACEtomo requires the setup of at least 2 image states in the SerialEM navigator.
- One image state for whole grid montages (LM maps), at a pixel size <400 nm/px (including binning).
- One Low Dose mode image state with a View magnification for lamella montages (MM maps), ideally at pixel sizes <2.2 nm/px and a defocus offset of 50-100 μm, as well as the desired Record settings for tilt series acquisition. The smaller the beam diameter in Record mode, the more targets can be selected per lamella.
Additionally, an intermediate magnification (IM) might be required to compensate for the coordinate shifts between the low mag and the View mag. This magnification should contain the lamella fully in the FOV when moving the stage to a position on the LM map and it should be fairly well aligned to the View mag.
Details
When selecting and moving to a point on an LM map, then taking a View image, the selected point will most likely not be in the field of view. Usually, I find that point manually and use the Navigator > Shift to Marker function to adjust the coordinates.To automate this, SPACEtomo moves to a lamella found on the LM map and takes an image at IM that needs to fully contain the lamella despite the offset. It then runs the same lamella detection model and shifts the coordinates to the newly found lamella. The jump from the IM to the View magnification in your Low Dose image state should be minimal since no further compensation is applied.
For optimal results, it is recommended to check or redo the "Mag IS Offsets" calibration for the relevant magnifications.
The magnifications I use are 82x for LM, 580x for IM and 4800x for MM.
If you intend to do a coma-free alignment and a coma vs image shift calibration, this should be done prior to running SPACEtomo ideally on a carbon support foil grid.
All settings are adjusted in the SPACEtomo.py script.
The most important settings for daily use are the targeting settings:
- The target_list includes all classes of the segmentation that are targeted. Examples for all classes can be found here.
- The avoid_list includes all classes that should be avoided.
- The target_score_threshold can be adjusted to reduce the number of targets. The score is calculated from the overlap of the camera field of view with the segmented classes. A desired class in the center of the camera is upweighted. Generally, larger targets (e.g. nucleus or cell) are more robust to higher thresholds.
- sparse_targets is a useful target selection mode for small targets like mitos or vesicles. If set to False, a rigid grid of points is used for initial target selection, which is more suited to large target areas.
- target_edge can be used to target the edges of a segmented class. This could be useful for membrane structure studies (e.g. NPCs).
- penalty_weight: Factor to downweight the classes of the avoid_list relative to the classes of the target_list.
- max_iterations: Maximum number of iterations for the target setup optimization.
- If you want to add an extra target for the tracking tilt series that does not contain your desired class you can set extra_tracking to True.
Workflow settings should remain mostly unchanged after being set once. They include the image state settings, aperture control, montage tile overlaps and thresholds.
- SPACE_DIR: Path to the SPACEtomo folder containing the models and scripts.
- automation_level: Level 1-5 from only taking the WG map to starting the PACEtomo batch acquisition.
- plot: Save plots of intermediate steps (mainly during target selection).
- aperture_control: Does SerialEM have control over the apertures?
- objective_aperture: Size of objective aperture to be inserted when leaving LM.
- WG_image_state: Image state index used for LM map
- MM_image_state: Image state index used for Low Dose mode tilt series acquisition.
- WG_offset_via_IM: Use an intermediate magnification to keep track of lamella when switching image states.
- IM_mag_index: The mag index (e.g. 10) of the intermediate magnification or the magnification itself (e.g. 580).
- MM_padding_factor: MM maps are padded by this factor compared to the lamella bounding box. This accounts for the coordinates being off center or the bounding box prediction being off.
- MM_mean_threshold: If the first View image of a lamella is below this threshold (black), the user will be prompted to manually adjust the position. Set to 0 to avoid a user prompt.
- WG_montage_overlap: Overlap between neighboring tiles of the LM map for stitching.
- MM_montage_overlap: Overlap between neighboring tiles of the MM map for stitching. Good stitching is especially important here for reliable target selection.
- WG_detection_threshold: YOLOv8 confidence threshold for considering a hit a lamella. Raise this value if you have a lot of false positives.
- max_tilt: This is the maximum tilt angle during a tilt series. It is used to calculate target spacing without any beam overlap. You can use lower values if you don't care about high angle overlaps and rather have more targets.
- tolerance: If two targets are overlapping each other below this threshold, they are moved away from each other. If the overlap is larger than this, one of them is deleted.
The external_map_dir setting can be used when running the segmentation inference on an external machine (see below).
The SPACEtomo_config.py only needs adjustment when updating the deep learning models.
You can run SPACEtomo at different automation levels. It will stop after reaching the final automation level and will let you manually finish the setup if you desire.
- SPACEtomo will load a grid, collect a LM map of the whole grid and identify the positions of lamellae. It will then take another image of a lamella at IM to adjust the stage coordinates accordingly. If you stop at level 1, you can manually move to the identified lamella and decide where you want to collect a montage or select targets using PACEtomo_selectTargets.py.
- This level includes collection of MM maps of each lamella (optionally include lamella identified as broken). You can then use these maps for manual target selection. Level 2 is organism independent and should be able to be used for any kind of lamella sample.
- Level 3 includes the subsequent segmentation of the lamella montages for manual inspection. This model is organism specific (only Yeast at time of release) and new models will be released in the future.
- If you intend to collect lamellae exhaustively, you can use the Yeast model and include "lamella" in the first entry of your target_list. This allows the target setup to use the organism independent classes (e.g. "black", "white", "ice", etc.) to avoid and set up targets everywhere else on the lamella.
- SPACEtomo will use the generated segmentation to set up targets according to your target selection settings. If you stop at level 4, you will be able to review all selected targets or add additional targets using the PACEtomo_selectTargets.py script.
- The highest level of automation will start SerialEM's Acquire at Items routine. Make sure to set it up accordingly in advance.
- You have to select the PACEtomo.py script as the Primary Action and make sure that the PACEtomo settings in the script have been set appropiately.
- Additionally, you have to set the SPACEtomo_postAction.py script as Run Script after Action. This script will monitor any finishing lamella segmentations that were not ready for target setup before the PACEtomo acquisition started.
- SPACEtomo will create a navigator file for each grid. Any kind of montages are saved according to SerialEM settings. Additionally, a plot of detected lamellae with assigned classes and probabilities is saved as <grid_name>_lamella_detected.png.
- All lamella montages are saved as image file rescaled to the pixel size of the segmentation model.
- Segmentations are saved with the suffix _seg for each MM map. Each segmentation inference prodcues a _SPACE.log file for debugging.
- The SPACE_runs.txt and SPACE_queue.txt files are necessary to schedule the inference jobs.
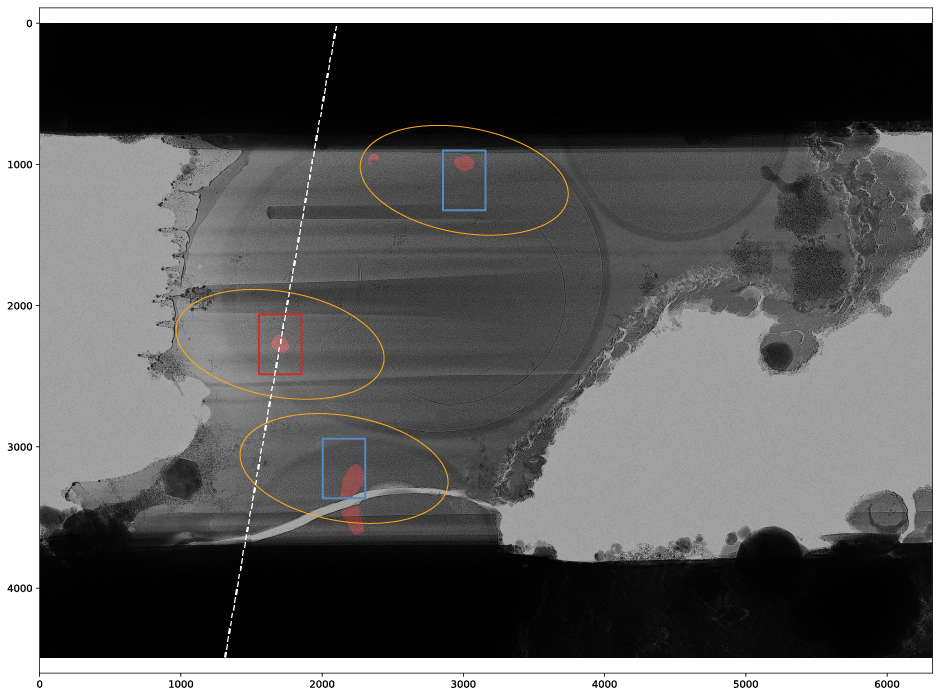
- Each lamella produces several plots for the target setup including the targets during each iteration of target position refinement and the final targets on the lamella with the mask overlay in red. Beam shapes at maximum tilt angle are marked by yellow ellipses. Camera fields of view are outlined in red for the tracking target and blue for other targets. The dashed white line represents the tilt axis orientation. Colored dots indicate clustering of positions.
- Tilt series are output as specified by SerialEM and PACEtomo.
Depending on your setup, the GPU on the computer running SerialEM might not be powerful enough to analyze MM maps as fast as you collect them. This is not a problem per se since the segmentation inference keeps running in the background during data collection. However, you will need the first segmentation to setup the initial batch of targets, which might cause waiting time in some cases.
To process MM maps on an external machine with a better GPU you can specify an external_map_dir in the settings of the SPACEtomo.py script. This directory has to be accessible via the network both by the computer running SerialEM and by the external GPU machine.
The external GPU machine requires the same Python packages as described above and a copy of the SPACEtomo folder (but no SerialEM). Open the SPACEtomo_monitor.py script and adjust the external_map_dir accordingly.
You can then simply activate the conda environment and run the monitor script:
conda activate SerialEM_pytorch
python <SPACEtomo_dir>/SPACEtomo_monitor.py
The monitor script will check the map dir periodically, queue any MM maps to be analayzed, and save the segmentations to the same directory.
SPACEtomo on the SerialEM computer will keep checking the map dir for new segmentations and setup the targets when appropiate.
Coming as soon as possible!
- In case your lamella montages don't stitch properly, you might need to experiment with the SerialEM montage settings.
- To be continued...
Release!
- Additional tools for manual corrections without interrupting the Run
- Creation of lamella maps with segmented target overlay for manual target picking
- Multi grid SPACEtomo runs
- Segmentation models for Chlamydomonas and eukaryotic cells
- Framework for training your own models
- To be continued... (Let me know if you have wishes or ideas!)
SPACEtomo training dataset for lamella detection using YOLOv8
SPACEtomo training dataset for Yeast lamella map segmentation using nnU-Netv2
There is no straight forward way yet to label your own data yet. For the input of a nnU-Net model you will need an image file and a segmentation file with a particular pixel value for each class. You can find further instructions here.
For my training set, I used Photoshop to segment different classes on different layers by hand. Why Photoshop? My main reason was the support for comfortable labeling using a drawing tablet with pressure sensitiviy. I saved each layer as png file separately and used a Python script to combine these images into a single segmentation image. Another script would take these segmentations and output layer images that I could then edit and refine in Photoshop for retraining.
Napari might be better suited for your case. OpenFIBSEM includes a Napari-based labelling workflow that can be adapted for nnU-Net.
My goal is to work on making a more streamlined labeling workflow similar to this in the future.
I want to thank all the people who provided training data for the deep learning models:
- Anna Bieber (MPI Martinsried)
- Cristina Capitanio (MPI Martinsried)
- Matthias Pöge (MPI Martinsried)
- Sven Klumpe (MPI Martinsried)
- Gregor Weiss (ETH Zurich)
- Yoshiyuki Fukuda (Tokushima University / University of Tokyo)