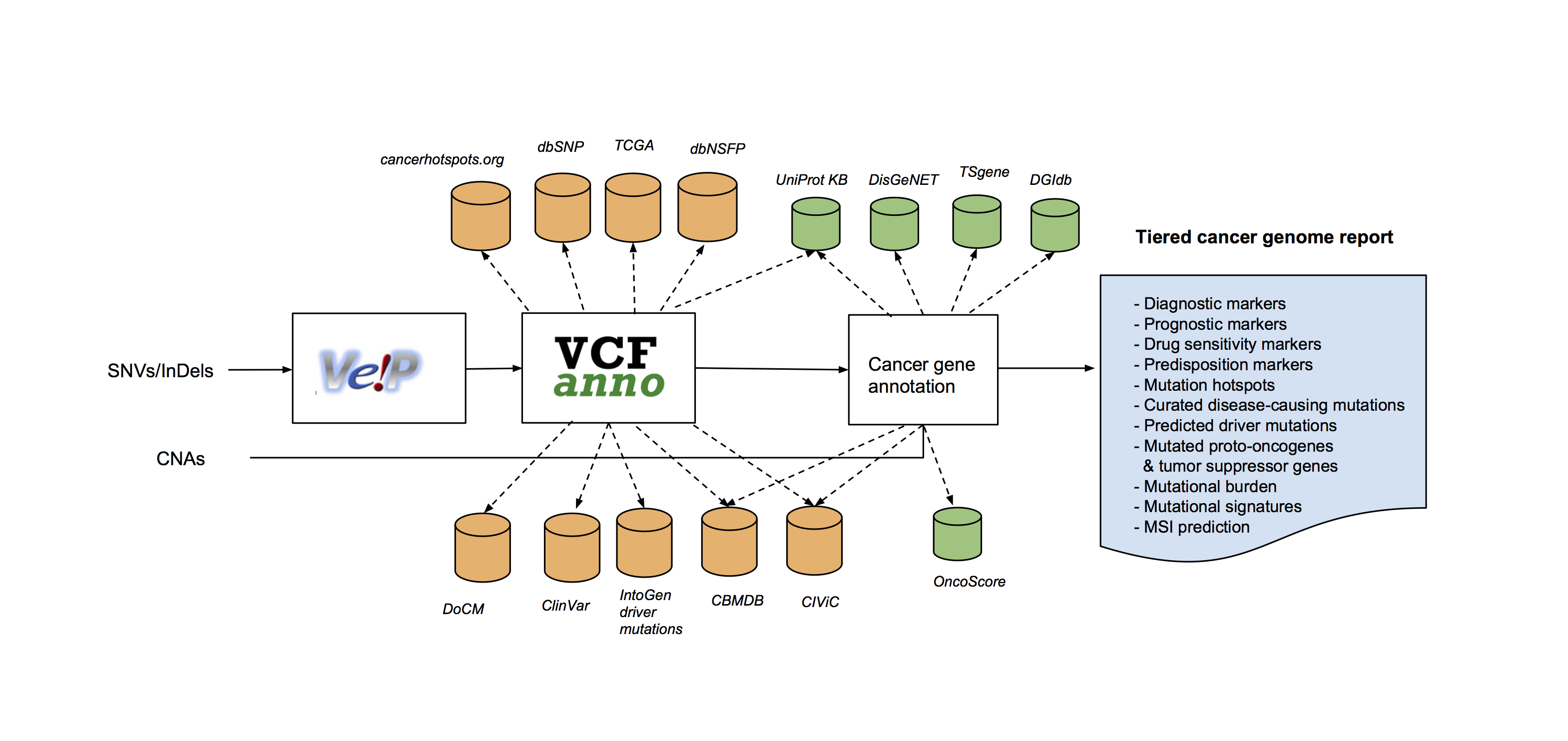
The Personal Cancer Genome Reporter (PCGR) is a stand-alone software package for functional annotation and translation of individual cancer genomes for precision oncology. It interprets both somatic SNVs/InDels and copy number aberrations. The software extends basic gene and variant annotations from the Ensembl’s Variant Effect Predictor (VEP) with oncology-relevant, up-to-date annotations retrieved flexibly through vcfanno, and produces interactive HTML reports intended for clinical interpretation (Figure 1).
- November 29th 2017: 0.5.3 release
- Fixed bug with propagation of default options
- November 23rd 2017: 0.5.2 release
- November 15th 2017: 0.5.1 pre-release
- Bug fixing (VCF validation)
- November 14th 2017: 0.5.0 pre-release
- Updated version of VEP (v90)
- Updated versions of ClinVar, Uniprot KB, CIViC, CBMDB
- Removal of ExAC (replaced by gnomAD), removal of COSMIC due to licensing restrictions
- Users can analyze samples run without matching control (i.e. tumor-only)
- PCGR pipeline is now configured through a TOML-based configuration file
- Bug fixes / general speed improvements
- Work in progress: Export of report data through JSON
If you use PCGR, please cite our recent publication:
Sigve Nakken, Ghislain Fournous, Daniel Vodák, Lars Birger Aaasheim, Ola Myklebost, and Eivind Hovig. Personal Cancer Genome Reporter: variant interpretation report for precision oncology (2017). Bioinformatics (in press). doi:10.1093/bioinformatics/btx817
- VEP v90 - Variant Effect Predictor release 90 (GENCODE v27 as the gene reference dataset)
- dBNSFP v3.4 - Database of non-synonymous functional predictions (March 2017)
- gnomAD r1 - Germline variant frequencies exome-wide (March 2017)
- dbSNP b147 - Database of short genetic variants (April 2016)
- 1000 Genomes Project - phase3 - Germline variant frequencies genome-wide (May 2013)
- TCGA release 9.0 - somatic mutations discovered across 33 tumor type cohorts (The Cancer Genome Atlas)
- ClinVar - Database of clinically related variants (November 2017)
- DoCM - Database of curated mutations (v3.2, April 2016)
- CIViC - Clinical interpretations of variants in cancer (November 11th 2017)
- CBMDB - Cancer Biomarkers database (November 11th 2017)
- IntOGen catalog of driver mutations - (May 2016)
- DisGeNET - Database of curated gene-tumor type associations (May 2017)
- Cancer Hotspots - Resource for statistically significant mutations in cancer (2016)
- UniProt/SwissProt KnowledgeBase 2017_10 - Resource on protein sequence and functional information (October 2017)
- Pfam v31 - Database of protein families and domains (March 2017)
- DGIdb - Database of targeted cancer drugs (v3.0, September 2017)
- TSGene v2.0 - Tumor suppressor/oncogene database (November 2015)
A local installation of Python (it has been tested with version 2.7.13) is required to run PCGR. Check that Python is installed by typing python --version in a terminal window. In addition, a Python library for parsing configuration files encoded with TOML is needed. To install, simply run the following command:
pip install toml
- Install the Docker engine on your preferred platform
- installing Docker on Linux
- installing Docker on Mac OS
- NOTE: We have not yet been able to perform enough testing on the Windows platform, and we have received feedback that particular versions of Docker/Windows do not work with PCGR (an example being mounting of data volumes)
- Test that Docker is running, e.g. by typing
docker psordocker imagesin the terminal window - Adjust the computing resources dedicated to the Docker, i.e.:
- Memory: minimum 5GB
- CPUs: minimum 4
- How to - Mac OS X
-
Download and unpack the latest software release (0.5.3)
-
Download and unpack the data bundle (approx. 16Gb) in the PCGR directory
- Download the accompanying data bundle from Google Drive to
~/pcgr-X.X(replace X.X with the version number, e.g~/pcgr-0.5.3) - Unpack the data bundle, e.g. through the following Unix command:
gzip -dc pcgr.databundle.GRCh37.YYYYMMDD.tgz | tar xvf -
A data/ folder within the pcgr-X.X software folder should now have been produced
- Download the accompanying data bundle from Google Drive to
-
Pull the PCGR Docker image (0.5.3) from DockerHub (approx 4.2Gb):
docker pull sigven/pcgr:0.5.3(PCGR annotation engine)
The PCGR workflow accepts two types of input files:
- An unannotated, single-sample VCF file (>= v4.2) with called somatic variants (SNVs/InDels)
- A copy number segment file
NOTE: GRCh37 is currently supported as the reference genome build
PCGR can be run with either or both of the two input files present.
- We strongly recommend that the input VCF is compressed and indexed using bgzip and tabix
- If the input VCF contains multi-allelic sites, these will be subject to decomposition
- Variants used for reporting should be designated as 'PASS' in the VCF FILTER column
The tab-separated values file with copy number aberrations MUST contain the following four columns:
- Chromosome
- Start
- End
- Segment_Mean
Here, Chromosome, Start, and End denote the chromosomal segment (GRCh37), and Segment_Mean denotes the log(2) ratio for a particular segment, which is a common output of somatic copy number alteration callers. Below shows the initial part of a copy number segment file that is formatted correctly according to PCGR's requirements:
Chromosome Start End Segment_Mean
1 3218329 3550598 0.0024
1 3552451 4593614 0.1995
1 4593663 6433129 -1.0277
A tumor sample report is generated by calling the Python script pcgr.py, which takes the following arguments and options:
usage: pcgr.py [-h] [--input_vcf INPUT_VCF] [--input_cna INPUT_CNA]
[--force_overwrite] [--version]
pcgr_dir output_dir configuration_file sample_id
Personal Cancer Genome Reporter (PCGR) workflow for clinical interpretation of
somatic nucleotide variants and copy number aberration segments
positional arguments:
pcgr_dir PCGR base directory with accompanying data directory,
e.g. ~/pcgr-0.5.3
output_dir Output directory
configuration_file PCGR configuration file (TOML format)
sample_id Tumor sample/cancer genome identifier - prefix for
output files
optional arguments:
-h, --help show this help message and exit
--input_vcf INPUT_VCF
VCF input file with somatic query variants
(SNVs/InDels). Note: GRCh37 is currently the only
reference genome build supported (default: None)
--input_cna INPUT_CNA
Somatic copy number alteration segments (tab-separated
values) (default: None)
--force_overwrite By default, the script will fail with an error if any
output file already exists. You can force the
overwrite of existing result files by using this flag
(default: False)
--version show program's version number and exit
The configuration file, formatted using TOML (an easy to read file format) enables the user to configure a number of options in the PCGR workflow, related to the following:
- MSI prediction
- Mutational signatures analysis
- Coding target size - for mutational burden analysis
- Tumor-only analysis options (i.e. exclusion of germline variants/enrichment for somatic calls)
- VEP/vcfanno options
- Specification of INFO tags in VCF that denote sequencing depth/allelic support of variants
- Log-ratio thresholds for gains/losses in CNA analysis
The examples folder contain input files from two tumor samples sequenced within TCGA. It also contains a PCGR configuration file. A report for a colorectal tumor case can be generated by running the following command in your terminal window:
python pcgr.py --input_vcf ~/pcgr-0.5.3/examples/tumor_sample.COAD.vcf.gz
--input_cna ~/pcgr-0.5.3/examples/tumor_sample.COAD.cna.tsv
~/pcgr-0.5.3 ~/pcgr-0.5.3/examples ~/pcgr-0.5.3/examples/pcgr_configuration_examples.toml tumor_sample.COAD
This command will run the Docker-based PCGR workflow and produce the following output files in the examples folder:
- tumor_sample.COAD.pcgr.html - An interactive HTML report for clinical interpretation
- tumor_sample.COAD.pcgr.vcf.gz - VCF file with rich set of annotations for precision oncology
- tumor_sample.COAD.pcgr.maf - A basic MAF file for use as input in downstream analyses with other tools (e.g. 2020plus, MutSigCV)
- tumor_sample.COAD.pcgr.snvs_indels.tiers.tsv - Tab-separated values file with variants organized according to tiers of functional relevance
- tumor_sample.COAD.pcgr.mutational_signatures.tsv - Tab-separated values file with estimated contributions by known mutational signatures and associated underlying etiologies
- tumor_sample.COAD.pcgr.snvs_indels.biomarkers.tsv - Tab-separated values file with clinical evidence items associated with biomarkers for diagnosis, prognosis or drug sensitivity/resistance
- tumor_sample.COAD.pcgr.cna_segments.tsv.gz - Tab-separated values file with annotations of gene transcripts that overlap with somatic copy number aberrations