#HiCPlotter: Visualization of Hi-C data with genomic datasets
HiCPlotter is a Python data visualization tool for integrating different data types with interaction matrixes. For more on 5C or Hi-C, please check: Dekker et al. 2013.
If you use HiCPlotter in your studies, please cite our publication in Genome Biology (http://www.genomebiology.com/2015/16/1/198)
HiCPlotter is designed by Kadir Akdemir (kcakedemir at mdanderson dot org / find me on Twitter) while in Lynda Chin's Lab at the University of Texas MD Anderson Cancer Center, Houston, TX, USA.
python HiCPlotter.py -f HUVEC.txt NHEK.txt -r 25000 -chr chr16 -n HUVEC NHEK -da 1 -o TOX3 -mm 6 -spi 1 -t HUVEC.E122.states.bed NHEK.E127.states.bed -tl States States -s 1940 -e 2156 -hist HUVEC.H3K9me3.bedGraph,Huvec.CTCF.bedGraph NHEK.H3K9me3.bedGraph,Nhek.CTCF.bedGraph -hl H3K9me3,CTCF H3K9me3,CTCF -si 1 -hc FF78B7,85d8e6 FF78B7,85d8e6 -fhist 1,0 1,0 -hm 18,600 18,600 -c 1 -a HUVEC.Arrowhead.txt NHEK.Arrowhead.txt -al Domains Domains -ac cecece cecece -g Sox9genes.sorted.bed
Python 2.7.*
- Please note: scipy, numpy and matplotlib modules should be installed and updated to current version. Following versions of numpy (1.9.0, 1.9.2, 1.10.4), scipy(0.14.0, 0.15.1, 0.17.0) and matplotlib(1.3.1, 1.4.3, 1.5.1) have been tested successfully.
- If you receive error(s) related to one or more of these modules, check this solution and/or check versions of python and required modules.
HiCPlotter is tested on Mac OS (Mountain Lion and Yosemite) and Linux (RedHat 4.1.2-44 and 5.5-Final) systems.
HiCPlotter is purposefully designed with the least amount of dependencies to make it easily applicable.
- Input Files
- Basic Usage
- Drawing Genes
- Tracks
- Highlights
- Annotation
- Whole Genome Plots
- 5C Plots
- Matrix Comparisons
For reading more about each parameter, please check the manual.
Required parameters:
files (-f) : a list of filenames to be plotted.
name (-n) : a list of labels for the experiment.
chr (-chr) : chromosome to be plotted.
output (-o) : prefix for the output file.
Optional parameters:
verbose (-v) : print version and arguments into a file.
tripleColumn (-tri) : a boolean if input file is from HiC-Pro pipeline.
dark (-da) : a boolean to use black background for the output.
bedFile (-bed) : a file name for bin annotations, if -tri parameter is set.
plotGenes (-g) : a sorted bed file for plotting the locations of the genes.
geneLabels (-gl) : a boolean for plotting gene labels (1:default) or not (0).
histograms (-hist) : a list of filenames to be plotted as histogram.
histLabels (-h) : a list of labels for the histograms.
fillHist (-fhist) : a list whether each histogram will be filled (1) or not (0:default).
histColors (-hc) : a list of hexadecimal numbers for histogram filling colors.
histMax (-hm) : a list of integer for maximum values of histograms.
superImpose (-si) : a boolean to overlap two histogram files inside the same track (default:0) enable(1).
start (-s) : retain after x-th bin (0:default).
end (-e) : continues until x-th bin (default: length of the matrix).
resolution (-r) : resolution of the bins (default: 100000).
matrixMax (-mm) : an integer value for the interaction matrix heatmap scale upper-limit.
barPlots (-b) : a list of filenames to be plotted as bar plots.
barLabels (-bl) : a list of labels for the bar plots.
barColors (-bc) : a list of hexadecimal numbers for coloring the bar plots.
barMax (-bm) : a list of integer for maximum values of bar plots.
tilePlots (-t) : a list of filenames to be plotted as tile plots.
tileLabels (-tl) : a list of labels for the tile plots.
tileColors (-tc) : a list of hexadecimal numbers for coloring the tile plots.
tileText (-tt) : a boolean whether text will be displayed above tiles (0:default) or not (1).
arcPlots (-a) : a list of filenames to be plotted as arc plots.
arcLabels (-al) : a list of labels for the arc plots.
arcColors (-ac) : a list of hexadecimal numbers for coloring the arc plots.
highlights (-high) : a boolean for enabling highlights on the plot (0:default), enable(1).
highFile (-hf) : a file name for a bed file to highlight selected intervals.
peakFiles (-peak) : a list of filenames to be plotted on the matrix.
compare (-c) : a boolean to plot log2 compare first two matrices (default:0) enable(1).
pair (-p) : a boolean to plot log2 pair-wise matrix comparisons (default:0) enable(1).
spine (-spi) : a boolean to remove top and left borders for each tracks (default:0) enable(1).
epiLogos (-ep) : a filename to be plotted as Epilogos format.
oExtension (-ext) : an extension name for the output file format - default jpeg.
imputed (-im) : a boolean if imputed epilogos will be plotted. (default:0 for observed)
window (-w) : an integer of distance to calculate insulation score.
tadRange (-tr) : an integer of window to calculate local minima for TAD calls.
fileHeader (-fh) : an integer for how many lines should be ignored in the matrix file (1:default).
fileFooter (-ff) : an integer for how many lines should be skipped at the end of the matrix file (0:default).
smoothNoise (-sn) : a floating-point number to clean noise in the data.
heatmapColor (-hmc) : an integer for choosing heatmap color codes: Greys(0), Reds(1), YellowToBlue(2), YellowToRed(3-default), Hot(4), BlueToRed(5).
cleanNANs (-cn) : a boolean for replacing NaNs in the matrix with zeros (1:default) or not (0).
plotTriangular (-ptr) : a boolean for plotting rotated half matrix (1:default) or not (0).
plotTadDomains (-ptd) : a boolean for plotting TADs identified by HiCPlotter (1) or not (0:default).
plotPublishedTadDomins (-pptd) : a boolean for plotting TADs from Dixon et, al. 2012 (1:default) or not (0).
plotDomainsAsBars (-ptdb) : a boolean for plotting TADs as bars (1) instead of triangles (0:default)
highResolution (-hR) : a boolean whether plotting high resolution (1:default) or not (0).
dPixels (-dpi) : an integer to determine dots per inch in matrix, higher values for higher resolution (default:200).
plotInsulation (-pi) : a boolean for plotting insulation scores (0:default) or plot (1).
randomBins (-rb) : a boolean for plotting random resolution data (1:default) or not (0).
wholeGenome (-wg) : a boolean for plotting whole genome interactions (1:default) or not (0).
plotCustomDomains (-pcd) : a list of file names to be plotted beneath the matrix.
domColors (-dc) : a list of hexadecimal numbers for coloring the domain plots.
publishedTadDomainOrganism (-ptdo) : a boolean for plotting human (1:default) or mouse (0) TADs from Dixon et, al. 2012.
customDomainsFile (-pcdf) : a list of filenames to be plotted as TADs for each experiments.
For visualizing Hi-C or 5C data, HiCPlotter requires a matrix file (by default first line is ignored).
Matrix files are plotted as their log2 values and color legend is put below the plot.
Bin1 Bin2 Bin3 Bin4 Bin5 Bin6
7.85957 4.80329 11.4766 9.57416 4.5288 8.55022
8.61621 4.98956 2.35654 5.69483 11.1187 10.1322
4.06803 4.07801 7.98047 2.59144 6.3851 7.74306
4.52869 2.70624 8.94544 4.29185 8.29491 8.38257
2.91472 3.84658 1.56752 4.48515 7.4955 8.77461
3.08096 2.96487 7.23623 2.33142 3.08529 5.5379
3.12141 3.06905 4.97247 2.39298 5.03621 7.22344
3.4037 2.26455 1.48176 1.41958 3.40252 7.7027
3.8696 1.41425 7.68872 2.21027 5.06846 3.20063
If your file (example is modified from GSM873926) has several header lines, use -fh parameter (this particular case -fh 5).
# resultName mESCs-female-PGK-day2-Replicate1
#
#
#
REV_2|mm9|chrX:98831149-98834145 REV_4|mm9|chrX:98837507-98840771 REV_6|mm9|chrX:98841228-98843248 REV_12|mm9|chrX:98855723-98862021
936.010581657246 743.499513378904 241.956223702097 23.2451328286973
69.8831429744098 513.412096905019 747.143877424081 7.1902317648089
If your file is in bed file format as in GSM1081531, you can covert it to a matrix file with bedToMatrix.py in Utils directory. Each file should have data from one chromosome. To split the file, you can use -> awk '{if($1=="chr6") print}' [file] > [newfile]
chr6 80000_120000 120000_160000 10.278
chr6 80000_120000 160000_200000 3.648
chr6 80000_120000 200000_240000 4.204
*run python bedToMatrix.py [file_name]
HiCPlotter now accepts the output format of HiC-Pro pipeline, where the matrix file is a three column sparse format in which first two columns are interacting bins and third column is interaction frequency. Bins do not interact with each other (with score 0) are not listed in the file.
1050 1586 1
1050 1589 1
1050 1590 1 (jumps to 1612)
1050 1612 2
An annotation file (bed format) is also required to denote each bin's chromosomal locations. Fourth column is the bin number inside the matrix file.
chr1 20960000 20980000 1049
chr1 20980000 21000000 1050
chr1 21000000 21020000 1051
chr1 21020000 21040000 1052
chr1 21040000 21060000 1053
chr1 21060000 21080000 1054
chr1 21080000 21100000 1055
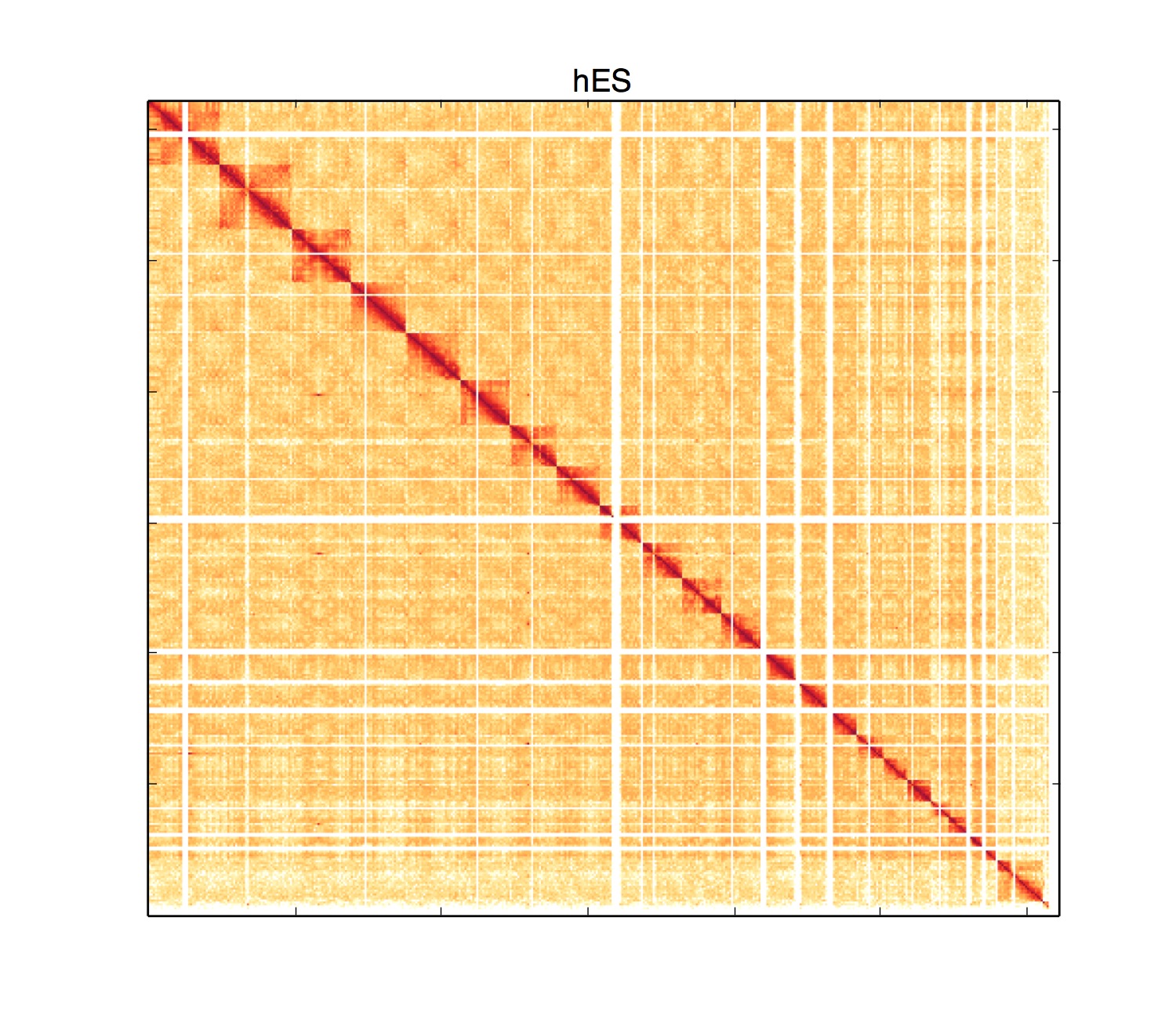
User should set -tri parameter to 1 and provide the bin-annotation bed file with -bed FILENAME. An example usage:
python HiCPlotter.py -f GSE35156_GSM892306_hESC.40000.matrix -chr chr7 -o Example -r 40000 -tri 1 -bed GSE35156_GSM892306_hESC.40000_ord.bed -n hES -s 650 -e 700
For visualizing any type of genomic data, HiCPlotter uses bedGraph format.
chromA chromStartA chromEndA dataValueA color (optional) text (optional)
chr1 10000 10500 10.0 250,13,27 Polycomb
4th column should be a floating number for histograms.
5th column should be an rgb color for tile or arc plots.
6th column should be a string for tile plots. Columns after 6th will be ignored.
For annotating the interaction matrix, HiCPlotter requires the following format.
chromA chromStartA chromEndA chromB chromStartB chromEndB color (optional)
chr10 100180000 100190000 chr10 100410000 100420000 0,255,255
chr10 101600000 101610000 chr10 101800000 101810000 0,255,255
chr10 102100000 102105000 chr10 102190000 102195000 0,255,255
For plotting genes under the interaction matrix, HiCPlotter requires the following format.
chrom geneStart geneEnd geneName strand(optional) exonStarts(optional) exonEnds(optional) color (optional) fontSize (optional)
chr1 11873 14409 DDX11L1 + 11873,12645,13220, 12227,12697,14409, 250,13,27 15
chr1 14361 16765 WASH7P - 14361,14969,15795,16606, 14829,15038,15942,16765,
chr1 14361 19759 WASH7P - 14361,14969,15795,16606,16857,17232,17605,17914,18267,18912, 14829,15038,15947,16765,17055,17368,17742,18061,18366,19759,
This file should be sorted based on 2nd column (geneStart).
To specify specific gene label size, provide an integer at column 9 (a color should be provided at column 8th)
For 6th (exonStarts) and 7th (exonEnds) columns, there should be a comma at the very end.
If 5th column is provided then 6th and 7th columns should be provided as well.
python HiCPlotter.py -f file1 file2 -n name1 name2 -chr chrX -o output
You can generate all of the following examples by using "sh testRun.sh", all of required files are in the data folder.
Please note: -fh is set to 0 as the input matrix doesn't have a header line.
Hi-C and TADs data taken from: Dixon et, al. Nature 2012
python HiCPlotter.py -f data/HiC/Human/hES-nij.chr21.2 -n hES -chr chr21 -r 40000 -o default1 -fh 0
Start and end locations can be specified as bin numbers with -s and -e parameters.
Color of triangles specify interaction frequency in a given TAD.
TADs identified by Dixon et al. can be plotted with -pptd parameter.
TADs can be plotted as bars instead of triangles with -pdb parameter.
python HiCPlotter.py -f data/HiC/Human/hES-nij.chr21.2 -n hES -chr chr21 -r 40000 -o default2 -ptd 1 -pptd 1 -s 600 -e 900 -fh 0 -w 8 -tr 10 -pi 1
python HiCPlotter.py -f data/HiC/Human/hES-nij.chr21.2 -n hES -chr chr21 -r 40000 -o default2 -ptd 1 -pptd 1 -s 600 -e 900 -fh 0 -w 8 -tr 10 -pi 1 -pdb 1
Hi-C data taken from: Zuin et, al. PNAS 2014
Color code of the heatmaps can be changed with -hmc parameter
python HiCPlotter.py -f data/HiC/Human/GSM1081526_TEV_r1_cis.index.chr6.txt_matrix.txt data/HiC/Human/GSM1081528_HRV_r1_cis.index.chr6.txt_matrix.txt data/HiC/Human/GSM1081530_CTRL_r1_cis.index.chr6.txt_matrix.txt data/HiC/Human/GSM1081533_CTCF_r2_cis.index.chr6.txt_matrix.txt -n WT RAD21-Depleted siControl CTCF-Depleted -chr chr6 -r 40000 -fh 0 -pi 0 -sn 0.35 -o Rad21.CTCF -s 2800 -e 2950 -hmc 5
Saving the output file as a pdf gives better output compared to default jpeg file.
python HiCPlotter.py -f data/HiC/Human/IMR90-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt -o genes -n IMR90 -chr chr17 -g genes.sorted.bed -r 25000 -s 1800 -e 1850 -hist data/HiC/Human/IMR90.Rad21.bedGraph -hl Rad21 -hm 500 -ext pdf
Multiple histograms for the same matrix should be seperated by comma (true for hist labels and fill histogram parameters).
Data taken from: 4C : Noordermer et, al. Elife 2014, Hi-C and TADs : Dixon et, al. Nature 2012 and CTCF : Stadler et, al. Nature 2011
python HiCPlotter.py -f data/HiC/Mouse/mES.chr2 -n mES -chr chr2 -r 40000 -o HoxD -hist data/HiC/Mouse/GSM1334415_4C_Mouse_EScells_Hoxd4_smoothed_11windows.bedGraph,data/HiC/Mouse/GSM1334440_4C_Mouse_E9.5TB_Hoxd4_smoothed_11windows.bedGraph,data/HiC/Mouse/GSM1334412_4C_Mouse_EScells_Hoxd13_smoothed_11windows.bedGraph,data/HiC/Mouse/GSM1334437_4C_Mouse_E9.5TB_Hoxd13_smoothed_11windows.bedGraph,data/HiC/Mouse/GSM747534_ChIPseq_CTCF_ES_rep1.chr2.bedGraph -hl Hoxd4-ES,Hoxd4-Tail,Hoxd13-ES,Hoxd13-Tail,CTCF-ES -s 1830 -e 1880 -fh 0 -pi 0 -pcd 1 -pcdf data/mES_domains_mm9.bed -fhist 1,1,1,1,0 -hm 2000,2000,2000,2000,50
Color for area under the curve fillings can be specific as a hexadecimal number with -hc parameter.
python HiCPlotter.py -f data/HiC/Mouse/mES.chr2 -n mES -chr chr2 -r 40000 -o HoxDc -hist data/HiC/Mouse/GSM1334415_4C_Mouse_EScells_Hoxd4_smoothed_11windows.bedGraph,data/HiC/Mouse/GSM1334412_4C_Mouse_EScells_Hoxd13_smoothed_11windows.bedGraph -hl Hoxd4-ES,Hoxd13-ES -s 1830 -e 1880 -fh 0 -pi 0 -pcd 1 -pcdf data/mES_domains_mm9.bed -fhist 1,1 -hm 2000,2000 -hc 143D52,9ACD32
To remove frames for each track, use --spine (-spi) 1 parameter.
python HiCPlotter.py -f data/HiC/Mouse/mES.chr2 -n mES -chr chr2 -r 40000 -o HoxDcs -hist data/HiC/Mouse/GSM1334415_4C_Mouse_EScells_Hoxd4_smoothed_11windows.bedGraph,data/HiC/Mouse/GSM1334412_4C_Mouse_EScells_Hoxd13_smoothed_11windows.bedGraph -hl Hoxd4-ES,Hoxd13-ES -s 1830 -e 1880 -fh 0 -pi 0 -pcd 1 -pcdf data/mES_domains_mm9.bed -fhist 1,1 -hm 2000,2000 -hc 143D52,9ACD32 -spi 1
Gene Expression data taken from: Plasschaert et, al. NAR 2014
Color can be specified as a hexadecimal number (-bc 9ACD32) or for each bar by specified RGB colors in bedGraph file.
python HiCPlotter.py -f data/HiC/Mouse/mES.chr6 -n mES -chr chr6 -r 40000 -o HoxDb -b data/HiC/Mouse/GSE39522_rnaseq_gene_expression.bedGraph -bl GeneExpression -s 2900 -e 3075 -fh 0 -pi 0 -bc 9ACD32 -bm 500 -mm 8 -spi 1
Arc plots require a bedGraph file (-a file1), color can be specified as a hexadecimal number (-ac B4B4B4) or for each arc by specified RGB colors in bedGraph file.
Data taken from: SMC ChIA-Pet and Polycomb Domains: Dowen et, al. Cell 2014, Hi-C and TADs : Dixon et, al. Nature 2012 and H3K27me3 : Mouse ENCODE Project
python HiCPlotter.py -f data/HiC/Mouse/mES.chr3 -n mES -chr chr3 -o Bhlhe22 -r 40000 -s 400 -e 500 -a data/HiC/Mouse/mESC_SMC_ChIPPet.bed -al SMC -hist data/HiC/Mouse/GSM747534_chr3.bedGraph,data/HiC/Mouse/wgEncodeLicrHistoneEsb4H3k27me3ME0C57bl6StdSig.chr3.bedGraph -hl CTCF,H3K27me3 -pi 0 -ptr 0 -t data/HiC/Mouse/mm9_Polycomb_domains.bed -tl Polycomb -tc 00CCFF -ac B4B4B4 -fh 0
If bedGraph file for tile plotting contains text in 6th column, features can be plotted above tiles with -tt parameter.
Data taken from: 4C : Lonfat et, al. Science 2014 (Primer locations in Lonfat et, al. are extended to make them more visible in the plot), Hi-C and TADs : Dixon et, al. Nature 2012
python HiCPlotter.py -f data/HiC/Mouse/mES.chr6 -n mES -chr chr6 -r 40000 -o Digit.vs.GT -s 1295 -e 1338 -hist data/HiC/Mouse/GSM1524258_segToFrag_4C_Digits_WT_E12-5_HoxA13_smoothed_11FragsPerWin.bedGraph,data/HiC/Mouse/GSM1524259_segToFrag_4C_GT_WT_E15-5_HoxA13_smoothed_11FragsPerWin.bedGraph -hl Digits,GT -fhist 1,1 -fh 0 -pi 0 -hm 1500,1500 -pcd 1 -pcdf data/mES_domains_mm9.bed -sn 0.4 -t data/HiC/Mouse/primers.bedGraph -tl Enhancers -tt 1
Epilogos is developed visualization and analysis of chromatin state model data in various cell types by Wouter Meuleman and Manolis Kellis. More about epilogos, check
You can download the epilogos data from
python HiCPlotter.py -f data/HiC/Human/GM12878-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt -chr chr10 -fh 0 -n GM12878 -o Epilogos -r 25000 -ep qcat -hist RepliSeq.bedGraph -hl RepliSeq -fhist 1 -s 2500 -e 5000 -mm 8
Use parameter (-im) if you download the qcat file from imputed/ folder
Currently color of each states for Epilogos plotting is hard-coded in HiCPlotter, therefore please use qcat files in imputed or observed folders.
Highlights on the plots can be drawn with -high 1 and passing a bed file name to -hf parameter.
Data taken from: Hi-C and Arrow domains : Rao et, al. Cell 2014, ChIA-Pet Heidari et, al. Genome Research 2014, TADs : Dixon et, al. Nature 2012, ChIP-Seq and Repli-Seq data : Encode Project
python HiCPlotter.py -f data/HiC/Human/GM12878-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 K562 -chr chr10 -r 25000 -pi 0 -fh 0 -o ChIA -a data/HiC/Human/GM12878.Rad21.bed data/HiC/Human/K562.Rad21.bed -al ChIA-PET ChIA-PET -s 3000 -e 3500 -pcd 1 -pcdf data/HiC/Human/GM12878_Arrowhead_domainlist.bed data/HiC/Human/K562_Arrowhead_domainlist.bed -hist data/HiC/Human/wgEncodeUwDnaseGm12878RawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneGm12878CtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqGm12878WaveSignalRep1.bedGraph data/HiC/Human/wgEncodeUwDnaseK562RawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneK562CtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqK562WaveSignalRep1.bedGraph -hl DNAse,CTCF,RepliSeq DNAse,CTCF,RepliSeq -fhist 0,0,1 0,0,1 -pptd 1 -high 1 -hf data/HiC/Human/highlight.bed
Annotations on the matrix can be drawn with -peak parameter.Input file should contain at least six columns.
Data taken from: Hi-C and HiCCUP peaks : Rao et, al. Cell 2014
python HiCPlotter.py -f data/HiC/Human/GM12878-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/KBM7-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/HUVEC-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/IMR90-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/HMEC-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/NHEK-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 KBM7 K562 HUVEC IMR90 HMEC NHEK -r 25000 -pi 0 -fh 0 -o Loops -chr chr10 -peak data/HiC/Human/GSE63525_GM12878_replicate_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_KBM7_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_K562_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_HUVEC_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_IMR90_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_HMEC_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_NHEK_HiCCUPS_looplist.bed -s 3600 -e 3675 -ptr 0
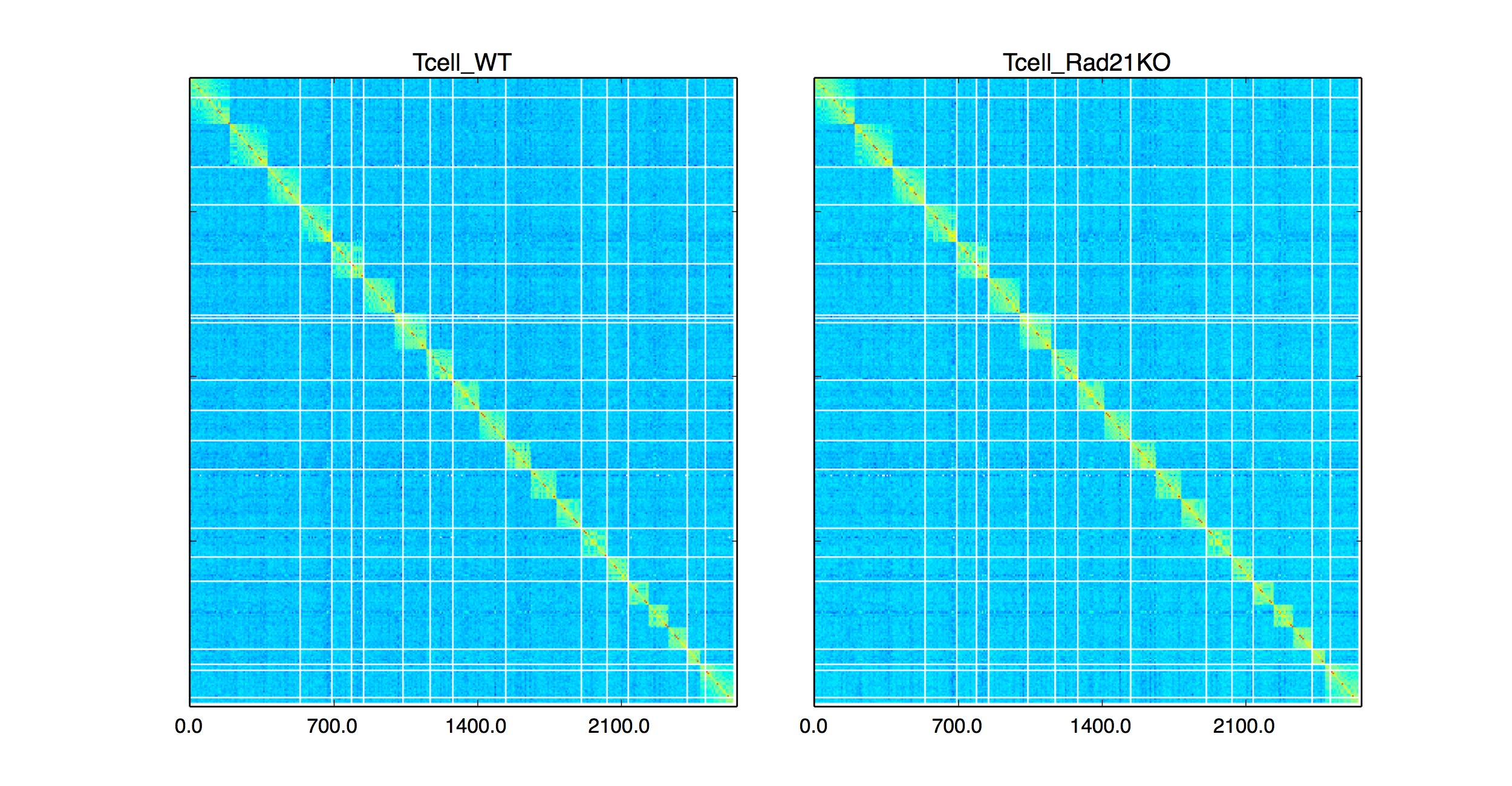
Whole genome plotting can be activated by -wg parameter.
Data taken from: Hi-C : Seitan et, al. Genome Research 2014
python HiCPlotter.py -f data/HiC/Human/GSM1184323-HiCMYZ-Tcell-Rad21WT-R1.mm9.NA.L-1400000-wDiag-noSS-iced.2.matrix data/HiC/Human/GSM1184321-HiCMYZ-Tcell-Rad21KO-R1.mm9.NA.L-1400000-wDiag-noSS-iced.2.matrix -n Tcell_WT Tcell_Rad21KO -chr Genome -r 1400000 -o Tcell -pi 0 -ptr 0 -wg 1 -hmc 5 -fh 4
(-chr) parameter will be used designate the end chromosome, such as (-chr chr11) will plot interactions starting from chr1 to chr11.
Please use (-chr chrY) for whole genome interaction plots.
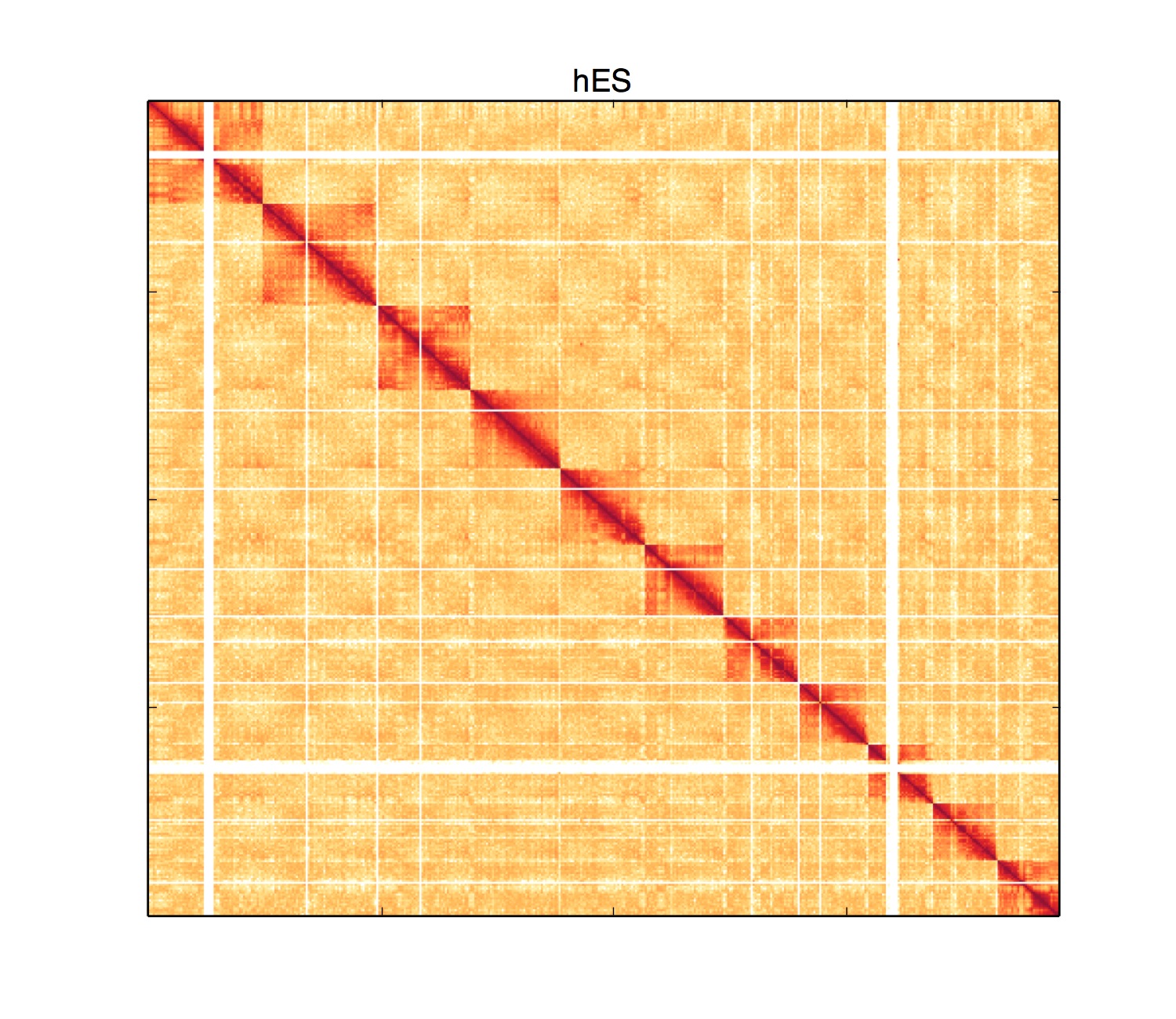
python HiCPlotter.py -f triple.sparse -tri 1 -bed triple.bed -wg 1 -chr chrY -o WholeGenome -n hES
python HiCPlotter.py -f triple.sparse -tri 1 -bed triple.bed -wg 1 -chr chr11 -o WholeGenome -n hES
Random binned 5C data plotting can be activated by -rb parameter (Please note: currently only matrixes and triangular plots can be plotted with this option).
Data taken from: 5C data Nora et, al. Nature 2012
python HiCPlotter.py -f data/5C/GSM873926_mESCs-female-PGK12.1-day2-Replicate1.txt data/5C/GSM873932_femaleXO-mESCs-DXTX-replicate-1.matrix.txt data/5C/GSM873924_female-MEFs-replicate-1.matrix.txt -n mESC mESC_XO MEF -fh 8 -chr chrX -o 5C -sn 2 -pi 0 -rb 1 -e 300 -hmc 5
Hi-C matrices can be compared by using -c parameter, which will generate log2 comparison matrix for the first two matrices.
python HiCPlotter.py -f data/HiC/Human/HUVEC-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/IMR90-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt -chr chr10 -n HUVEC IMR90 -o comparison -s 4850 -e 5400 -mm 8 -r 25000 -c 1 -t data/HiC/Human/HUVEC_18_core_K27ac_dense2.bed data/HiC/Human/IMR90_18_core_K27ac_dense2.bed -tl States States -spi 1 -hist data/HiC/Human/wgEncodeUwRepliSeqHuvecWaveSignalRep1.bedGraph data/HiC/Human/wgEncodeUwRepliSeqImr90WaveSignalRep1.bedGraph -hl RepliSeq RepliSeq -fhist 1 1
For pair-wise matrix comparisons in addition to -c parameter, also use -p parameter which will plot comparison matrices for each pair of matrices.
Warning: can be memory inefficient for high-resolution matrices.
python HiCPlotter.py -f HUVEC-chr21_25kb.RAWobserved_KRnormalizedMatrix.txt NHEK-chr21_25kb.RAWobserved_KRnormalizedMatrix.txt HMEC-chr21_25kb.RAWobserved_KRnormalizedMatrix.txt -n HUVEC NHEK HMEC -chr chr21 -r 25000 -o pair -c 1 -p 1 -spi 1 -mm 8 -s 650 -e 1210 -t HUVEC.E122.states.bed NHEK.E127.states.bed HMEC.E119.states.bed -tl States States States
python HiCPlotter.py -f data/HiC/Human/GM12878-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/HUVEC-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/NHEK-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/IMR90-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 K562 HUVEC NHEK IMR90 -chr chr10 -r 25000 -s 3000 -e 3250 -o Figure1 -fh 0
python HiCPlotter.py -f data/HiC/Human/GM12878-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/HUVEC-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/NHEK-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/IMR90-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 K562 HUVEC NHEK IMR90 -chr chr10 -r 25000 -s 3000 -e 3500 -o Figure2 -hist data/HiC/Human/wgEncodeUwDnaseGm12878RawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneGm12878CtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqGm12878WaveSignalRep1.bedGraph data/HiC/Human/wgEncodeUwDnaseK562RawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneK562CtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqK562WaveSignalRep1.bedGraph data/HiC/Human/wgEncodeUwDnaseHuvecRawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneHuvecCtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqHuvecWaveSignalRep1.bedGraph data/HiC/Human/wgEncodeUwDnaseNhekRawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneNhekCtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqNhekWaveSignalRep1.bedGraph data/HiC/Human/wgEncodeOpenChromDnaseImr90BaseOverlapSignal.chr10.bedGraph,data/HiC/Human/wgEncodeSydhTfbsImr90CtcfbIggrabSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqImr90WaveSignalRep1.bedGraph -fh 0 -fhist 0,0,1 0,0,1 0,0,1 0,0,1 0,0,1 -hl DNAse,CTCF,RepliSeq DNAse,CTCF,RepliSeq DNAse,CTCF,RepliSeq DNAse,CTCF,RepliSeq DNAse,CTCF,RepliSeq -hm 400,600,100 400,600,100 400,600,100 400,600,100 400,600,100 -pcd 1 -pcdf data/HiC/Human/GM12878_Arrowhead_domainlist.bed data/HiC/Human/K562_Arrowhead_domainlist.bed data/HiC/Human/HUVEC_Arrowhead_domainlist.bed data/HiC/Human/NHEK_Arrowhead_domainlist.bed data/HiC/Human/IMR90_Arrowhead_domainlist.txt -t data/HiC/Human/GM12878_18_core_K27ac_dense2.bed data/HiC/Human/K562_18_core_K27ac_dense2.bed data/HiC/Human/HUVEC_18_core_K27ac_dense2.bed data/HiC/Human/NHEK_18_core_K27ac_dense2.bed data/HiC/Human/IMR90_18_core_K27ac_dense2.bed -tl ChromHMM ChromHMM ChromHMM ChromHMM ChromHMM -pptd 1 -high 1 -hf data/HiC/Human/fig2.bed
python HiCPlotter.py -f data/HiC/Human/GM12878-chr15_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr15_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 K562 -chr chr15 -r 25000 -s 1800 -e 2250 -o Figure3 -hist data/HiC/Human/wgEncodeUwDnaseGm12878RawRep1.chr15.bedGraph,data/HiC/Human/wgEncodeBroadHistoneGm12878CtcfStdSig.chr15.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqGm12878WaveSignalRep1.bedGraph data/HiC/Human/wgEncodeUwDnaseK562RawRep1.chr15.bedGraph,data/HiC/Human/wgEncodeBroadHistoneK562CtcfStdSig.chr15.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqK562WaveSignalRep1.bedGraph -fh 0 -fhist 0,0,1 0,0,1 -hl DNase,CTCF,RepliSeq DNase,CTCF,RepliSeq -hm 400,400,100 400,400,100 -t data/HiC/Human/GM12878_Enhancer.bed,data/HiC/Human/GM12878_Txn.bed,data/HiC/Human/GM12878_Het.bed data/HiC/Human/K562_Enhancer.bed,data/HiC/Human/K562_Txn.bed,data/HiC/Human/K562_Het.bed -tl Enhancer,Transcribed,Heterochromatin Enhancer,Transcribed,Heterochromatin -a data/HiC/Human/GM12878.Rad21.bed data/HiC/Human/K562.Rad21.bed -al RAD21 RAD21 -ptr 0 -high 1 -hf data/HiC/Human/fig3.bed
python HiCPlotter.py -f data/HiC/Human/GM-chr19_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr19_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 K562 -r 25000 -chr chr19 -hist data/HiC/Human/GM12878.DNAse.chr19.2.bedGraph,data/HiC/Human/GM12878.RnaSeq.chr19.2.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqGm12878WaveSignalRep1.bedGraph data/HiC/Human/K562.DNAse.chr19.2.bedGraph,data/HiC/Human/K562.RnaSeq.chr19.2.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqK562WaveSignalRep1.bedGraph -hl DNAse,RNASeq,RepliSeq DNAse,RNASeq,RepliSeq -t data/HiC/Human/GM12878_TSS+Trx.2.bed data/HiC/Human/K562_TSS+Trx.2.bed -tl ChromHMM ChromHMM -high 1 -hf data/HiC/Human/region.bed -o Figure4 -s 1100 -e 1302 -hm 300,300,100 300,300,100 -fh 0 -fhist 0,0,1 0,0,1 -ptr 0
*If your data contains several columns before data matrix, from command line you could use:
cut -f N- matrix > new_matrix (where N is the ith column data values start)
*If any of the imported packages are missing in your python system, try to comment out those lines. For example:
Original : from scipy.signal import argrelextrema (line 20)
Try this : #from scipy.signal import argrelextrema (line 20). Use HiCPlotter with the -pi 0 and -ptd 0
*If you like to run HiCPlotter in verbose mode, please use -v parameter which will create a log file with which parameters the program ran.
*If you need to convert bigWig files to bedGraph files, you can use kentUtils/bigWigToBedGraph executable.
If you encounter any problems, please contact with - Kadir Akdemir (kcakedemir at mdanderson dot org).
The MIT License (MIT)
Copyright (c) 2015, Kadir C. Akdemir and Lynda Chin
Permission is hereby granted, free of charge, to any person obtaining a copy of this software and associated documentation files (the "Software"), to deal in the Software without restriction, including without limitation the rights to use, copy, modify, merge, publish, distribute, sublicense, and/or sell copies of the Software, and to permit persons to whom the Software is furnished to do so, subject to the following conditions:
The above copyright notice and this permission notice shall be included in all copies or substantial portions of the Software.
THE SOFTWARE IS PROVIDED "AS IS", WITHOUT WARRANTY OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO THE WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NONINFRINGEMENT. IN NO EVENT SHALL THE AUTHORS OR COPYRIGHT HOLDERS BE LIABLE FOR ANY CLAIM, DAMAGES OR OTHER LIABILITY, WHETHER IN AN ACTION OF CONTRACT, TORT OR OTHERWISE, ARISING FROM, OUT OF OR IN CONNECTION WITH THE SOFTWARE OR THE USE OR OTHER DEALINGS IN THE SOFTWARE.
Thanks to Lynda Chin and Andy Futreal for their leadership, management and support.
Thanks to Zeynep Coban-Akdemir, Ian Watson, Denise Spring, Jason Ernst, Tony Gutschner, Kunal Rai and Samir Amin for their insightful comments.
We are grateful to many researchers cited above for providing their data in publicly available and easy-to-use format.
.25K.jpeg)
.40K.jpeg)
.40K.jpeg)
.40K.jpeg)
.40K.jpeg)
.25K.jpeg)
.40K.jpeg)
.Colored.40K.jpeg)
.Frameless.40K.jpeg)
.40K.jpeg)
.40K.jpeg)
.40K.jpeg)
.25K.jpeg)
.25K.jpeg)
.25K.jpeg)
.RandomBins.jpeg)
.25K.jpeg)
.25K.jpeg)
.25K.jpeg)
.25K.jpeg)
.25K.jpeg)
.25K.jpeg)