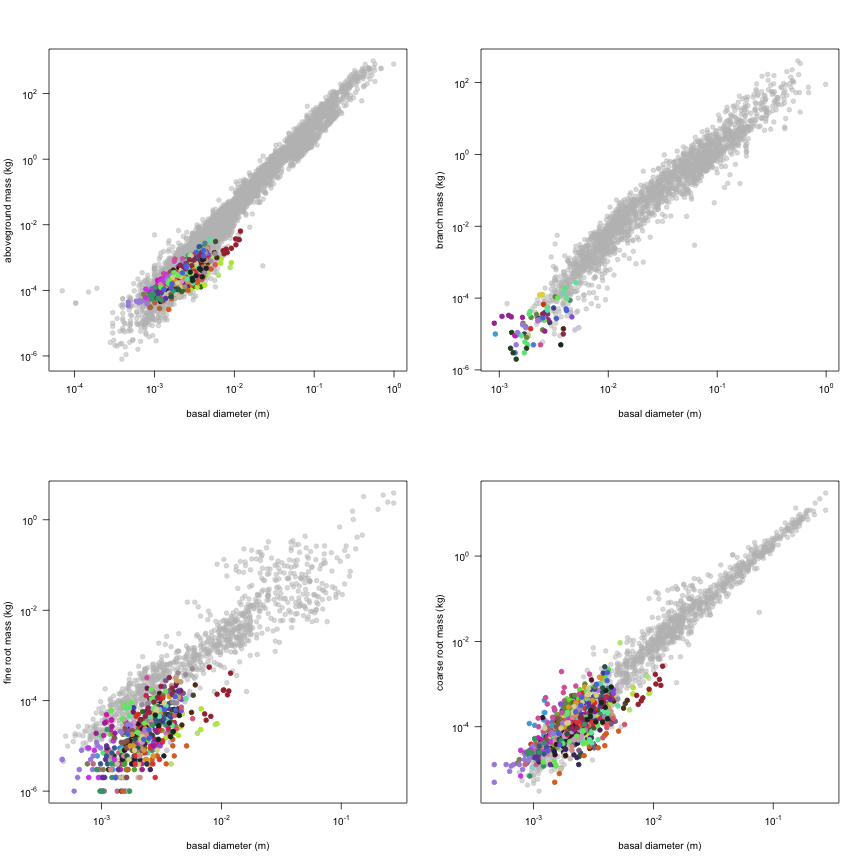-
Notifications
You must be signed in to change notification settings - Fork 19
Markesteijn2009
Data contributor: Lars Markesteijn, Lourens Poorter
Email: [email protected], [email protected]
Address:
- Department of Zoology, University of Oxford, South Parks Road, Oxford OX1, 3PS, UK
- Forest Ecology and Forest Management group, Wageningen University, P.O. Box 47, 6700 AA Wageningen, The Netherlands
Citation: Markesteijn L and Poorter L (2009). 'Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance.' Journal of Ecology, 97(2), pp. 311-325.
DOI: 10.1111/j.1365-2745.2008.01466.x
Abstract: * 1 Water availability is the main determinant of species' distribution in lowland tropical forests. Species' occurrence along water availability gradients depends on their ability to tolerate drought. * 2 To identify species' traits underlying drought-tolerance we excavated first year seedlings of 62 dry and moist forest tree species at the onset of the dry season. We evaluate how morphological seedling traits differ between forests, and whether functional groups of species can be identified based on trait relations. We also compare seedling traits along independent axes of drought and shade-tolerance to assess a hypothesized trade-off. * 3 Seedlings of dry forest species improve water foraging capacity in deep soil layers by an increased below-ground biomass allocation and by having deep roots. They minimize the risk of cavitation by making dense stems, and reduce transpiration by producing less leaf tissue. Moist forest seedlings have large leaf areas and a greater above-ground biomass, to maximize light interception, and long, cheap, branched root systems, to increase water and nutrient capture. * 4 Associations among seedling traits reveal three major drought strategies: (i) evergreen drought-tolerant species have high biomass investment in enduring organs, minimize cavitation and minimize transpiration to persist under dry conditions; (ii) drought-avoiding species maximize resource capture during a limited growing season and then avoid stress with a deciduous leaf habit in the dry season; (iii) drought-intolerant species maximize both below- and above-ground resource capture to increase competitiveness for light, but are consequently precluded from dry habitats. * 5 We found no direct trade-off between drought- and shade-tolerance, because they depend largely on different morphological adaptations. Drought-tolerance is supported by a high biomass investment to the root system, whereas shade-tolerance is mainly promoted by a low growth rate and low SLA. * 6 Synthesis. We conclude that there are three general adaptation strategies of drought-tolerance, which seemingly hold true across biomes and for different life forms. Drought- and shade-tolerance are largely independent from one another, suggesting a high potential for niche differentiation, as species' specialization can occur at different combinations of water and light availability.
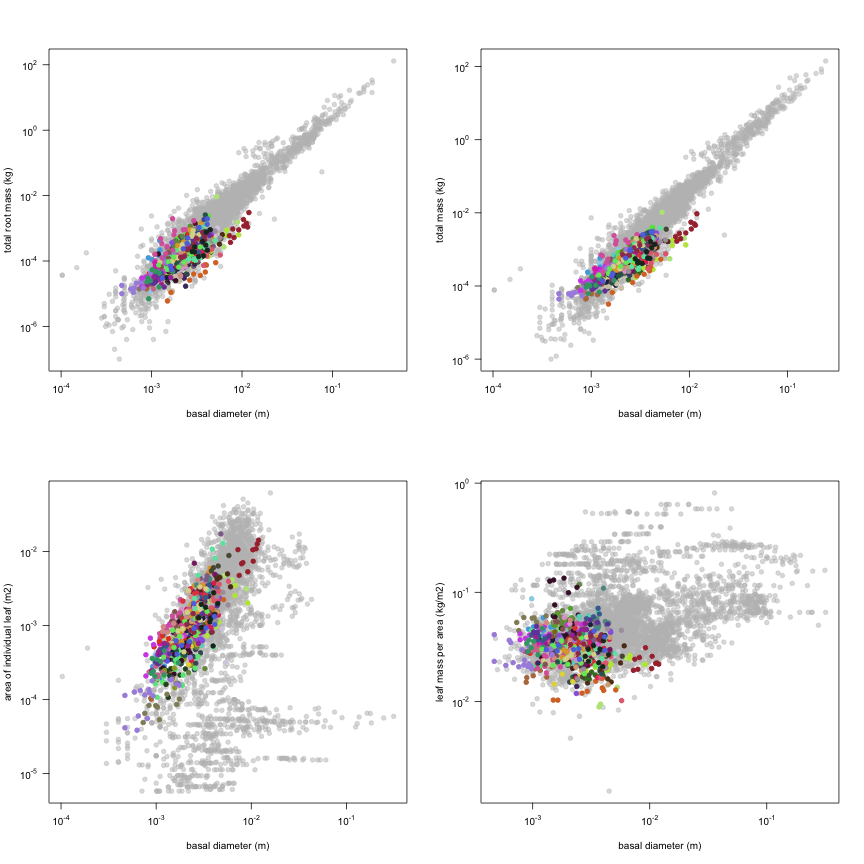
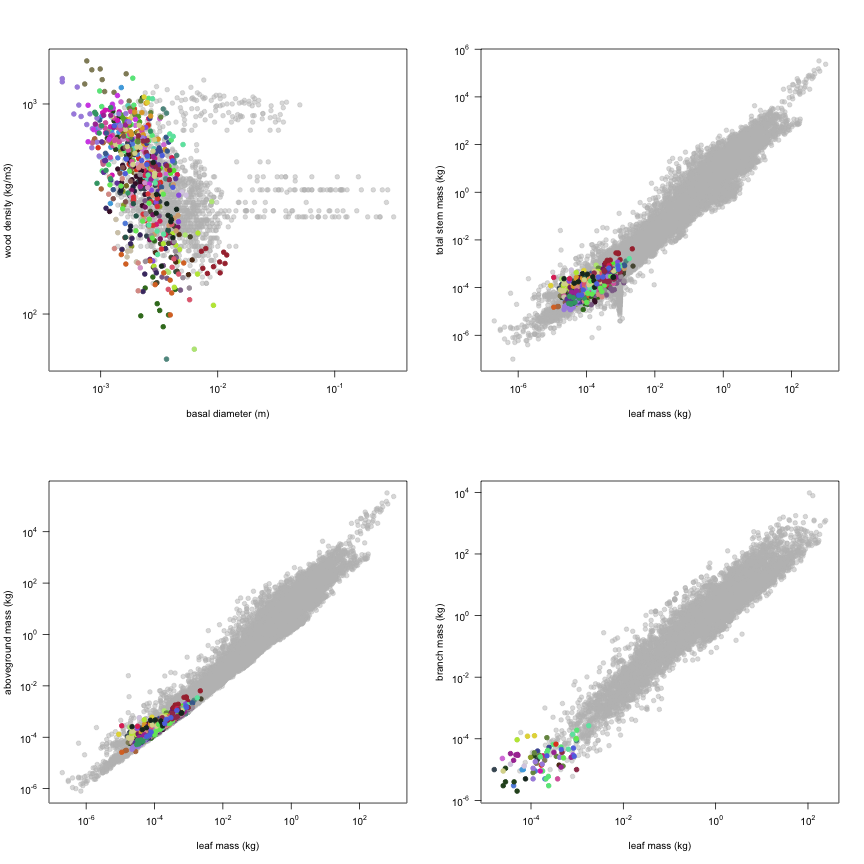
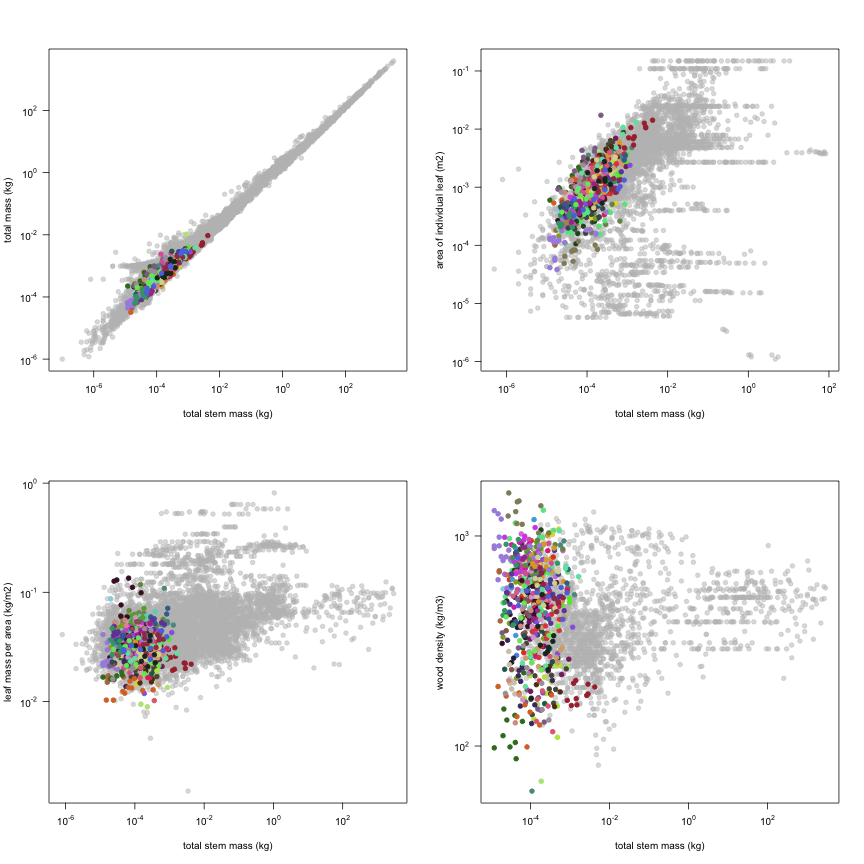
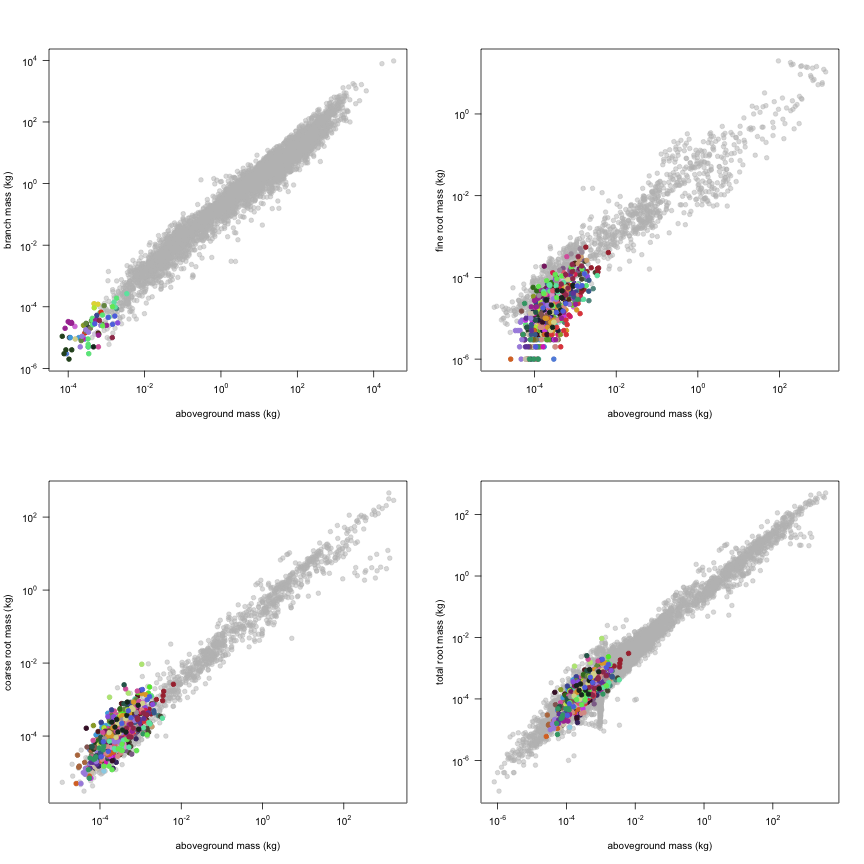
The dataset includes records for 662 individuals from 61 species belonging to 33 family(ies), presenting 2 functional type(s), growing in 1 condition(s) within 1 major type(s) of habitat, with data included for the following variables:
| Variable | Label | Units | N | Min | Median | Max |
|---|---|---|---|---|---|---|
| latitude | Latitude | deg | 662 | -16 | -16 | -16 |
| longitude | Longitude | deg | 662 | -62 | -62 | -62 |
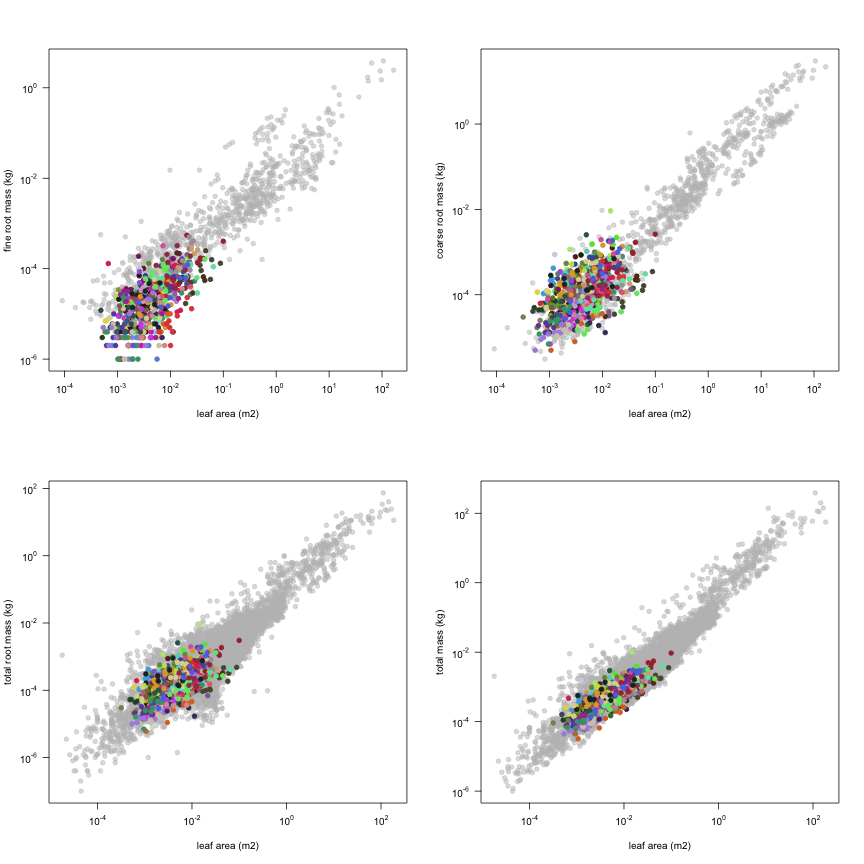
| a.lf | Leaf area | m2 | 662 | 0.00032 | 0.0044 | 0.1 |
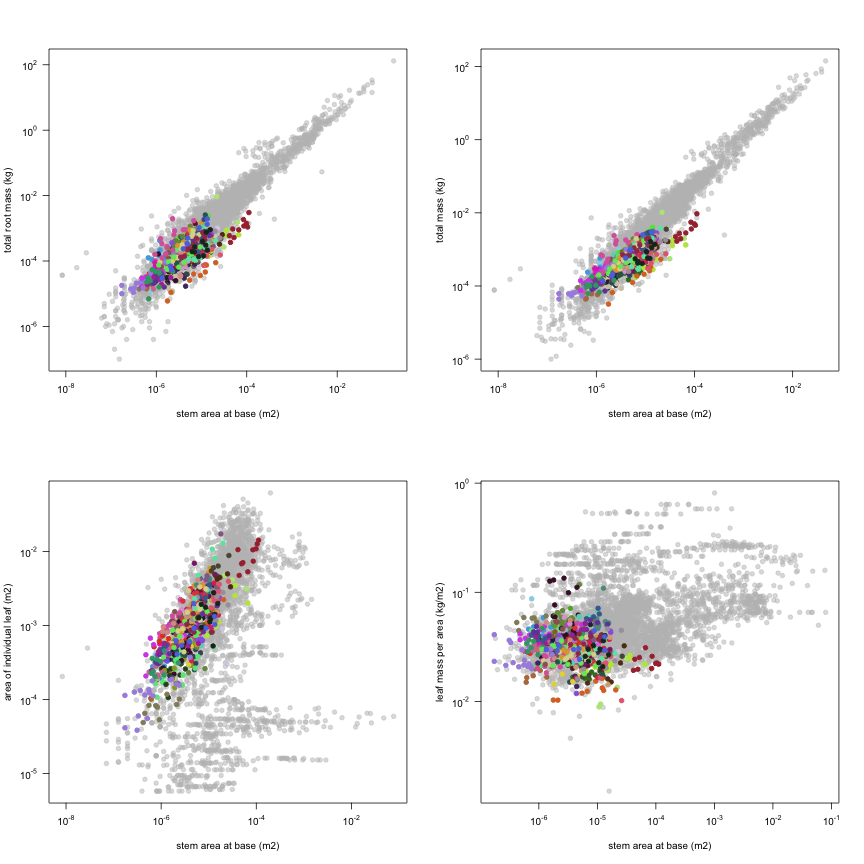
| a.stba | Stem area at base | m2 | 661 | 0.00000017 | 0.0000036 | 0.00011 |
| a.stbc | Stem area at crown base | m2 | 661 | 0.000000006 | 0.00000079 | 0.00002 |
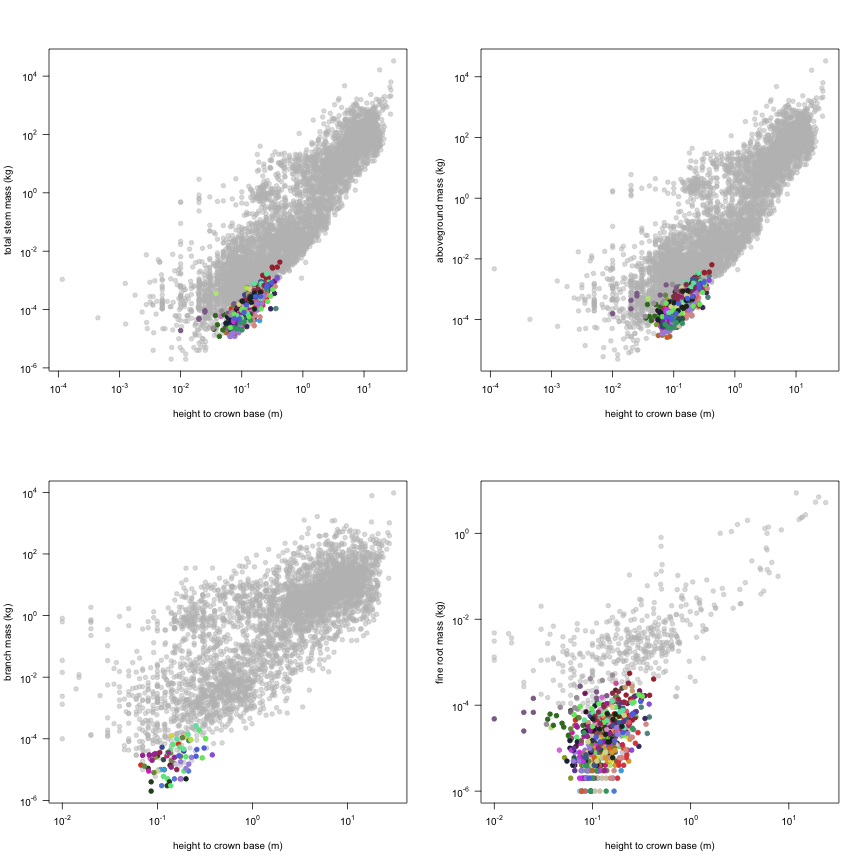
| h.c | Height to crown base | m | 661 | 0.01 | 0.12 | 0.42 |
| d.ba | Basal diameter | m | 661 | 0.00047 | 0.0021 | 0.012 |
| m.lf | Leaf mass | kg | 649 | 0.000009 | 0.00014 | 0.0023 |
| m.st | Total stem mass | kg | 647 | 0.000012 | 0.00013 | 0.0042 |
| m.so | Aboveground mass | kg | 649 | 0.000026 | 0.00027 | 0.0064 |
| m.br | Branch mass | kg | 75 | 0.000002 | 0.000025 | 0.00027 |
| m.rf | Fine root mass | kg | 586 | 0.000001 | 0.000019 | 0.00055 |
| m.rc | Coarse root mass | kg | 635 | 0.000005 | 0.00012 | 0.0093 |
| m.rt | Total root mass | kg | 647 | 0.000006 | 0.00014 | 0.0093 |
| m.to | Total mass | kg | 649 | 0.000032 | 0.00044 | 0.01 |
| a.ilf | Area of individual leaf | m2 | 662 | 0.000038 | 0.00084 | 0.017 |
| ma.ilf | Leaf mass per area | kg m-2 | 649 | 0.0089 | 0.031 | 0.13 |
| r.st | Wood density | kg m-3 | 646 | 61 | 506 | 1603 |
And locally within the country:

The sites sampled are:
| Location | Longitude | Latitude | Vegetation |
|---|---|---|---|
| Inpa | -61.72 | -16.12 | Tropical seasonal forest |
| La Chonta | -61.72 | -16.12 | Tropical seasonal forest |
The growing conditions of sampled plants was:
| Location | growingCondition |
|---|---|
| Inpa | field wild |
| La Chonta | field wild |
| Species | Family | Pft |
|---|---|---|
| Gallesia integrifolia | Phytolaccaceae | evergreen angiosperm, deciduous angiosperm |
| Pterogyne nitens | Fabaceae - Caesalpinioideae | evergreen angiosperm, deciduous angiosperm |
| Ampelocera ruizii | Ulmaceae | evergreen angiosperm, deciduous angiosperm |
| Combretum leprosum | Combretaceae | evergreen angiosperm, deciduous angiosperm |
| Jacaratia sp. | Caricaceae | evergreen angiosperm, deciduous angiosperm |
| Rollinia herzogii | Annonaceae | evergreen angiosperm, deciduous angiosperm |
| Erythroxylum daphnites | Erythroxylaceae | evergreen angiosperm, deciduous angiosperm |
| Bougainvillea modesta | Nyctanginaceae | evergreen angiosperm, deciduous angiosperm |
| Anadenanthera colubrina | Fabaceae - Mimosoideae | evergreen angiosperm, deciduous angiosperm |
| Casearia gossypiosperma | Flacourtiaceae | evergreen angiosperm, deciduous angiosperm |
| Phyllostylon rhamnoides | Ulmaceae | evergreen angiosperm, deciduous angiosperm |
| Simira rubescens | Rubiaceae | evergreen angiosperm, deciduous angiosperm |
| Myrciaria cauliflora | Myrtaceae | evergreen angiosperm, deciduous angiosperm |
| Aspidosperma tomentosum | Apocynaceae | evergreen angiosperm, deciduous angiosperm |
| Aspidosperma cylindrocarpon | Apocynaceae | evergreen angiosperm, deciduous angiosperm |
| Sweetia fruticosa | Fabaceae - Papilionoideae | evergreen angiosperm, deciduous angiosperm |
| Neea cf. steinbachii | Nyctanginaceae | evergreen angiosperm, deciduous angiosperm |
| Ceiba samaura | Malvaceae - Bombacoideae | evergreen angiosperm, deciduous angiosperm |
| Caesalpinia pluviosa | Fabaceae - Caesalpinioideae | evergreen angiosperm, deciduous angiosperm |
| Machaerium acutifolium | Fabaceae - Papilionoideae | evergreen angiosperm, deciduous angiosperm |
| Zanthoxylum monogynum | Rutaceae | evergreen angiosperm, deciduous angiosperm |
| Spondias mombin | Anacardiaceae | evergreen angiosperm, deciduous angiosperm |
| Capparis prisca | Capparaceae | evergreen angiosperm, deciduous angiosperm |
| Hymenaea courbaril | Fabaceae - Caesalpinioideae | evergreen angiosperm, deciduous angiosperm |
| Urera baccifera | Urticaceae | evergreen angiosperm, deciduous angiosperm |
| Pogonopus tubulosus | Rubiaceae | evergreen angiosperm, deciduous angiosperm |
| Psidium sartorianum | Myrtaceae | evergreen angiosperm, deciduous angiosperm |
| Trichilia elegans | Meliaceae | evergreen angiosperm, deciduous angiosperm |
| Copaifera chodatiana | Fabaceae - Caesalpinioideae | evergreen angiosperm, deciduous angiosperm |
| Solanum riparium | Solanaceae | evergreen angiosperm, deciduous angiosperm |
| Tabebuia impetiginosa | Bignoniaceae | evergreen angiosperm, deciduous angiosperm |
| Centrolobium microchaete | Fabaceae - Papilionoideae | evergreen angiosperm, deciduous angiosperm |
| Acosmium cardenasii | Fabaceae - Papilionoideae | evergreen angiosperm, deciduous angiosperm |
| Ceiba speciosa | Malvaceae - Bombacoideae | evergreen angiosperm, deciduous angiosperm |
| Stylogyne ambigua | Myrsinaceae | evergreen angiosperm, deciduous angiosperm |
| Pourouma cecropiifolia | Urticaceae | evergreen angiosperm, deciduous angiosperm |
| Cecropia concolor | Urticaceae | evergreen angiosperm, deciduous angiosperm |
| Heliocarpus americanus | Malvaceae - Tilioideae | evergreen angiosperm, deciduous angiosperm |
| Ficus boliviana | Moraceae | evergreen angiosperm, deciduous angiosperm |
| Cavanillesia hylogeiton | Malvaceae - Bombacoideae | evergreen angiosperm, deciduous angiosperm |
| Cedrela fissilis | Meliaceae | evergreen angiosperm, deciduous angiosperm |
| Alibertia verrucosa | Rubiaceae | evergreen angiosperm, deciduous angiosperm |
| Pouteria nemorosa | Sapotaceae | evergreen angiosperm, deciduous angiosperm |
| Jacaratia spinosa | Caricaceae | evergreen angiosperm, deciduous angiosperm |
| Sapindus saponaria | Sapindaceae | evergreen angiosperm, deciduous angiosperm |
| Ocotea sp. | Laureaceae | evergreen angiosperm, deciduous angiosperm |
| Sapium glandulosum | Euphorbiaceae | evergreen angiosperm, deciduous angiosperm |
| Pouteria macrohylla | Sapotaceae | evergreen angiosperm, deciduous angiosperm |
| Swietenia macrophylla | Meliaceae | evergreen angiosperm, deciduous angiosperm |
| Batocarpus amazonicus | Moraceae | evergreen angiosperm, deciduous angiosperm |
| Ocotea sp. 2 | Lauraceae | evergreen angiosperm, deciduous angiosperm |
| Licaria triandra | Lauraceae | evergreen angiosperm, deciduous angiosperm |
| Hura crepitans | Euphorbiaceae | evergreen angiosperm, deciduous angiosperm |
| Pseudolmedia laevis | Moraceae | evergreen angiosperm, deciduous angiosperm |
| Urera caracassana | Urticaceae | evergreen angiosperm, deciduous angiosperm |
| Hirtella triandra | Chrysobalanaceae | evergreen angiosperm, deciduous angiosperm |
| Schizolobium parahyba | Fabaceae - Caesalinioideae | evergreen angiosperm, deciduous angiosperm |
| Trema micrantha | Ulmaceae | evergreen angiosperm, deciduous angiosperm |
| Terminalia oblonga | Combretaceae | evergreen angiosperm, deciduous angiosperm |
| Cariniana ianeirensis | Lecythidaceae | evergreen angiosperm, deciduous angiosperm |
| Cariniana estrellensis | Lecythidaceae | evergreen angiosperm, deciduous angiosperm |
Sampling strategy: Seedlings were excavated at the onset of the dry season (April-May) to evaluate seedling morphology just before they were exposed to drought for the first time.
Leaf area: The number of leaves was counted and leaves were digitized with a desktop-scanner (Canon Lide 30). Total leaf area was determined with the help of pixel-counting software (Van Berloo 1998).
Stem cross sectional area: Diameters were recorded at the base and the top of the stem.
Height: Height measured from the base of the stem just above the root to the top of the stem just under the growth meristem.
Biomass: Seedlings were dissected into roots, stems, leaves weighed and then oven-dried for 48 h at 65degC and measured again for their dry mass.
Traits: Stem diameters at the base and top of the stem were used to calculate stem volume, which in turn together with stem mass generated an averaged stem density.
Year collected: 2006
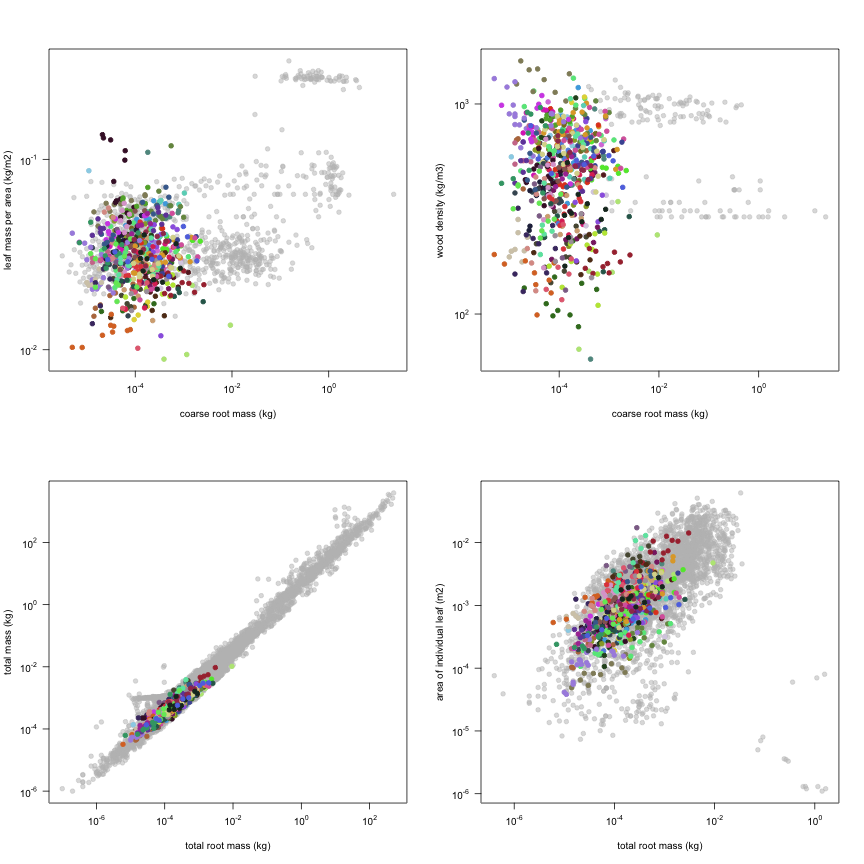
This is how the study Markesteijn2009 fits in the entire dataset (grey). each colour represents a species. A legend of species names with colours is included at the end for reports with 1 < n < 20 species.